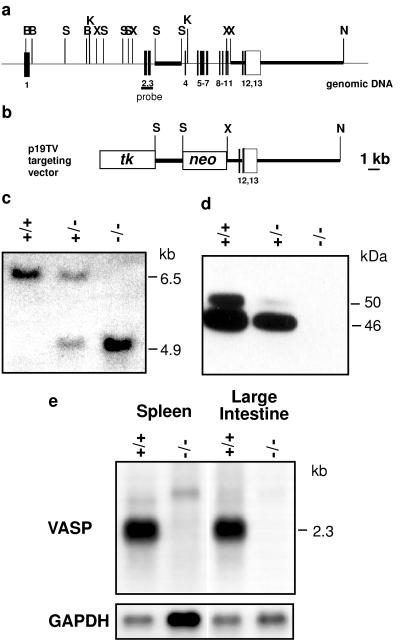
Figure 3.
Targeted disruption of the VASP gene. (a) Organization of the wild-type VASP gene and positions of relevant restriction sites (B, BamHI; K, KpnI; N, NheI; S, SacI; X, XhoI). Closed bars represent VASP coding exons, the open box indicates the 3′ untranslated region. Exons are numbered underneath. Thick lines represent the genomic fragments that were subcloned for construction of the targeting vector. The probe for Southern blot hybridization spans exons 2 and 3. (b) Targeting vector. The neomycin resistence gene (neo) was introduced in an antisense orientation with respect to the VASP gene. tk, thymidine kinase gene. (c) Southern blot analysis using DNA extracted from tail biopsies of wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice. DNA was digested with XhoI. Hybridization with the probe encompassing exons 2 and 3 yielded bands of 6.5 kb (wild-type allele) and 4.9 kb (mutant allele) in size. (d) Western blot analysis of VASP expression in platelets derived from wild-type, heterozygous, and mutant animals. The 46-kDa and 50-kDa proteins represent the serine-157 dephospho- and phospho-forms of VASP, respectively. (e) Northern blot analysis of poly(A)+ RNA from spleen and large intestine, wild-type and VASP−/− mice. The blots were hybridized with a human VASP full-length cDNA probe. For comparison of RNA amounts loaded the blots were hybridized with a mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe.