Abstract
Background
Little is known about the factors associated with frequency of emergency department visits (FEDV) in chronic obstructive pulmonary disease (COPD) patients with recurrent exacerbations.
Objective
To characterize the use of emergency department (ED) services in patients with COPD exacerbation and identify factors associated with FEDV.
Design
A prospective, multicenter cohort study.
Patients
Three hundred eighty-eight patients were included. Fifty-two percent were women and the median age was 69 years (interquartile range 62–76).
Measurements
Using a standard questionnaire, consecutive ED patients with COPD exacerbation were interviewed. The number of ED visits in the previous year was retrospectively collected.
Results
Over the past year, this cohort reported a total of 1,090 ED visits because of COPD exacerbation. Thirteen percent of COPD patients had 6 or more ED visits, accounting for 57% of the total ED visits in the past year. Multivariate analysis showed that patients with an increased FEDV were more likely to be Hispanic (incidence rate ratio [IRR] 1.97, 95% confidence interval [CI] 1.16–3.33), to have more severe COPD as determined by previous hospitalizations (IRR 2.06, 95% CI 1.51–2.82), prior intubations (IRR 1.49, 95% CI 1.02–2.18), prior use of systemic corticosteroids (IRR 1.57, 95% CI 1.16–2.13) and methylxanthine (IRR 1.48, 95% CI 1.04–2.12), and less likely to have a primary care provider (IRR 0.51, 95% CI 0.31–0.82).
Conclusions
Our results suggest that both disease and health care-related factors were associated with FEDV in COPD exacerbation. Multidisciplinary efforts through primary care provider follow-up should be assessed to test the effects on reducing the high morbidity and cost of recurrent COPD exacerbations.
KEY WORDS: chronic obstructive pulmonary disease, emergency department visits, primary care provider, recurrent exacerbation
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) affects more than 10 million people and is the fourth leading cause of death in the United States.1 It continues to cause a tremendous health and economic burden, with estimated annual costs in the United States of $24 billion in 2000.2 COPD exacerbations contribute to a significant proportion of this burden, resulting in approximately 1.5 million emergency department (ED) visits per year.1
It has been suggested that frequent COPD exacerbations are key drivers of hospital and other unscheduled care costs.3,4 Many COPD patients repeatedly return to hospital EDs for urgent COPD care, leading to rising health care expenditures. Evidence suggests that patients with frequent COPD exacerbations have a poorer quality of life,5 a faster decline in lung function,6 and increased mortality.7 Although exacerbation frequency is now recognized as an importance factor for morbidity and mortality in COPD, to our knowledge, no studies to date have examined the determinants of the frequency of emergency department visits (FEDV) for COPD exacerbation. Most studies focused on the factors associated with hospitalization for COPD exacerbation,8–10 but did not directly address this important topic. Understanding the determinants of FEDV may help identify interventions that reduce the high costs by frequent ED visits and subsequent morbidity and mortality in the patient population.
The primary purpose of this prospective multicenter study was to characterize the use of ED services in patients with COPD exacerbation and identify factors associated with FEDV in this patient population.
METHODS
Study Population
This observational study combined data from two identical prospective cohort studies performed in 2000–2001 as part of the Multicenter Airway Research Collaboration, a division of the Emergency Medicine Network (EMNet, www.emnet-usa.org). Details of the study design and data collection have been previously published.11 In brief, using a standard protocol, investigators at 29 EDs in 15 U.S. states and 3 Canadian provinces provided 24-hours-a-day coverage for a median of 2 weeks. Repeat visits by individual subjects were excluded. All patients were managed at the discretion of the treating physician. Inclusion criteria were physician diagnosis of COPD; presenting to the ED for treatment of COPD exacerbation, as defined by increasing shortness of breath, worsening cough, or change in sputum production;12,13 age ≥55 years; and the ability to give informed consent. The institutional review boards at each of the 29 participating hospitals approved the study, and informed consent was obtained for all participants.
Data Collection and Processing
Trained research personnel performed the ED interview and assessed patients’ baseline clinical characteristics, COPD history, and details of their current COPD exacerbation. Data on ED management and disposition were obtained from chart review. Site investigators reviewed all forms before submission to the EMNet Coordinating Center in Boston, where they underwent further review by trained personnel and then double data entry.
Information on Outcome and Covariates
Each patient’s diagnostic group was based on the following question: “Has a doctor ever said that you have asthma, COPD, or chronic bronchitis?” Patients were assigned to the COPD group if they reported COPD, emphysema, or chronic bronchitis. Patients reporting asthma and fulfilling the definition of COPD were classified as having both COPD and asthma (i.e., mixed disease). To address possible diagnostic misclassification, we performed a validation study in a subset of study population using information from spirometry, chest radiograph, and chest computed tomography scan, and 82% of self-reported cases of COPD were confirmed.14
The primary outcome of this study, number of ED visits, was based on the following question: “Over the past 12 months, how many times have you gone to a hospital ED because of your COPD?” The current visit was not counted. Median family income was estimated using home ZIP codes.15,16 Insurance status was categorized as private (commercial or private), Medicaid, other public (all Canadian and Medicare), or none. Primary care provider (PCP) status was assigned on the basis of the following question: “Do you have a primary care provider (such as a family doctor, internist, or nurse practitioner)?” Smoking status was coded as never a smoker, past smoker, or current smoker.
Statistical Analysis
Data are presented as proportions or medians with interquartile ranges (IQR) because all continuous variables were not normally distributed. We used negative binomial regression model to examine the univariate and multivariate factors associated with number of ED visits in the past year. We chose this model for two reasons. First, because there is no widely accepted definition of frequent ED visits, this model has advantage that there is no need to define an arbitrary cutoff point. Second, this model has been shown to better fit to numbers of ED visits by accounting for statistical overdispersion and excess zeros (individual with no ED visits) and yields more conservative results.17,18
Manual stepwise, multivariate negative binomial regression modeling was performed to assess factors associated with FEDV. Variables associated with FEDV at P < .10 in univariate analyses were considered eligible for inclusion in the multivariate analysis. Age, sex, race, education, and insurance status were included throughout the model building process because of their potential clinical significance. If deletion of a particular variable from the model changed any rate ratios in the existing model by less than 10%, this variable was deleted because it did not show a strong confounding effect on existing variables.19 We tested for two-way interaction by multiplying the two factors of interest and including an interaction term in the final multivariate model. Likelihood ratio tests were used to examine interactions with the comparison of nested models with and without the interaction variable. Incidence rate ratios (IRRs) are presented with 95% confidence intervals (CI).
We also calculated the population attributable risk (PAR) conferred by PCP status to estimate the percentage of ED visits in our cohort that, theoretically, would not have occurred if all COPD patients had a PCP, assuming a causal relationship between PCP and FEDV.20 This was calculated on the basis of the formula PAR = (p[IRR − 1] / (p[IRR − 1] + 1) × 100, where p is the prevalence of the population who lacked a PCP and IRR is the multivariate-adjusted IRR for lack of PCP. All analyses were performed using Stata version 9.0 software (StataCorp, College Station, Tex, USA). All P values are two-sided, with P < .05 considered statistically significant.
We performed two sensitivity analyses. First, to assess the impact of influential observations, we refitted the model by excluding two patients reporting more than 30 ED visits to see how the estimates changed in the final multivariate model. Second, to separate the potentially differential impact of insurance status on FEDV among Canadian patients (n = 63), we also refitted the model by excluding Canadian patients.
RESULTS
Of 853 eligible patients, 579 (65%) were enrolled with an exacerbation of asthma or COPD; analysis was restricted to the 397 patients with physician-diagnosed COPD (mixed asthma–COPD or COPD only). Enrolled and nonenrolled patients were similar across several sociodemographic factors (data not shown) except age. Nonenrolled patients were slightly older than enrolled patients (73 vs 70 years, P < .001). For the purpose of this analysis, we excluded 9 patients who had missing data on number of ED visits, leaving 388 patients for the current analysis.
The median age of these ED patients was 69 years and 52% were women (Table 1). Most participants were white and reported having a PCP and health insurance. About half reported using the ED as their usual site for problem COPD care, and 15% reported using the ED as their usual site for COPD prescriptions. More than 90% of patients reported a history of smoking, with an average of 51.5 pack-years among past and current smokers. Forty-four percent of patients reported having mixed COPD and asthma. Approximately two thirds of patients had been hospitalized for COPD and had taken corticosteroid for COPD; 14% of patients had been intubated for COPD. The use of multiple COPD medications also indicated the disease burden and severity in these elderly patients. Notably, comorbidities of cardiovascular diseases and depression were commonly seen in this population.
Table 1.
Demographic and baseline clinical characteristics of 388 emergency department (ED) patients with COPD exacerbation and the corresponding univariate analyses with respect to the frequency of ED visits for COPD exacerbation during the past year
| Characteristic | N = 388 | IRR | 95% Confidence interval | P value |
|---|---|---|---|---|
| Demographic factors | ||||
| Age (years)* | 69 (62–76) | 0.98 | 0.96–0.99 | 0.006 |
| Female sex | 200 (52) | 0.75 | 0.56–1.01 | 0.06 |
| Race | ||||
| White | 273 (70) | 1 | Reference | |
| Black | 82 (21) | 1.83 | 1.30–2.58 | <0.001 |
| Hispanic | 27 (7) | 3.50 | 2.07–5.92 | <0.001 |
| Others | 6 (2) | 1.78 | 0.59–5.38 | 0.31 |
| High school graduate | 206 (54) | 0.71 | 0.53–0.96 | 0.02 |
| Household income ($)* | 37231 (29319–46596) | 0.99 | 0.99–0.99 | <0.001 |
| Body mass index* | 24.9 (21.0–29.3) | 1.01 | 0.99–1.03 | 0.27 |
| Health care-related factors | ||||
| Insurance status | ||||
| Private | 92 (24) | 1 | Reference | |
| Medicaid | 56 (15) | 2.11 | 1.33–3.36 | 0.002 |
| Other public | 193 (51) | 0.89 | 0.62–1.28 | 0.53 |
| None | 39 (10) | 1.87 | 1.11–3.16 | 0.02 |
| Has primary care provider | 351 (90) | 0.41 | 0.26–0.66 | <0.001 |
| ED usual site for problem COPD care | 200 (53) | – | ||
| ED usual site for COPD prescriptions | 55 (15) | – | ||
| Smoking-related factors | ||||
| Never a smoker | 34 (9) | 1 | Reference | |
| Current smoker | 118 (30) | 1.39 | 0.78–2.47 | 0.27 |
| Past smoker | 236 (61) | 1.62 | 0.94–2.79 | 0.09 |
| Pack-years of smoking* | 51.5 (30–80) | 1.00 | 0.99–1.00 | 0.56 |
| Diagnosis | ||||
| COPD only | 218 (56) | 1 | Reference | |
| Mixed COPD and asthma | 170 (44) | 2.13 | 1.60–2.83 | <0.001 |
| Markers of chronic COPD severity | ||||
| Duration of COPD history (years) | 8.3 (3.5–19.6) | 0.99 | 0.99–1.01 | 0.76 |
| Breathing between COPD exacerbation*† | 1.02 | 0.89–1.16 | 0.77 | |
| No symptoms | 70 (18) | |||
| Some symptoms on some days | 112 (29) | |||
| Some symptoms on most days | 87 (23) | |||
| Symptoms most of the time | 117 (30) | |||
| Has a written plan for exacerbation | 45 (12) | 1.03 | 0.65–1.63 | 0.90 |
| Ever admitted for COPD | 252 (65) | 2.14 | 1.57–2.92 | <0.001 |
| Ever intubation for COPD | 53 (14) | 1.95 | 1.30–2.93 | 0.001 |
| Ever taken systemic corticosteroid for COPD | 254 (65) | 1.70 | 1.25–2.32 | <0.001 |
| COPD medications in past 4 weeks | ||||
| Inhaled β-agonists | 323 (83) | 1.89 | 1.26–2.84 | 0.002 |
| Inhaled anticholinergics | 246 (64) | 1.07 | 0.78–1.5 | 0.68 |
| Inhaled corticosteroid | 192 (49) | 1.17 | 0.87–1.57 | 0.30 |
| Systemic corticosteroid | 143 (37) | 1.99 | 1.49–2.67 | 0.001 |
| Other COPD medication | ||||
| Methylxanthine | 65 (17) | 2.30 | 1.60–3.32 | <0.001 |
| Home oxygen | 96 (25) | 1.06 | 0.75–1.48 | 0.75 |
| Long-acting Inhaled β-agonist | 60 (16) | 1.20 | 0.80–1.80 | 0.37 |
| Antibiotics | 69 (18) | 1.22 | 0.83–1.78 | 0.31 |
| Comorbidity | ||||
| Coronary artery disease | 86 (22) | 1.06 | 0.74–1.50 | 0.76 |
| Congestive heart failure | 71 (18) | 1.03 | 0.70–1.50 | 0.89 |
| History of arrhythmia | 49 (13) | 0.84 | 0.54–1.31 | 0.44 |
| Depression | 48 (12) | 1.53 | 0.99–2.37 | 0.05 |
| Any cancer (besides nonmelanoma skin cancer) | 44 (11) | 0.78 | 0.49–1.25 | 0.30 |
| Alcoholism | 22 (6) | 1.55 | 0.84–2.88 | 0.16 |
| Renal failure requiring dialysis | 3 (1) | 1.55 | 0.30–7.90 | 0.60 |
All values expressed as median (interquartile range) or percentage.
Chronic obstructive pulmonary disease (COPD)
*Incidence rate ratio (IRR) is for per one unit increase in the independent variable.
†On 4-point ordinal scale. (1, no symptoms; 4, with symptoms most of the time).
Over the past year, this cohort utilized a total of 1,090 ED visits because of COPD exacerbation. The median number of ED visits per person during the past year was 1 (IQR 0–3). Figure 1 shows the number of patients and the number of ED visits during the prior year, by the 4 frequency groups. One hundred thirty-eight patients (36%) reported no prior ED visits, 122 patients (31%) had 1 to 2 visits, 77 patients (20%) had 3 to 5 visits, and 51 patients (13%) had 6 or more visits in the past year. Relatively few frequent ED users were responsible for a disproportionately large number of the total ED visits. Patients with 1 to 2 visits during the past 12 months accounted for 17% of all ED visits; patients with 3 to 5 visits accounted for 27% of total visits; and patients with 6 or more visits accounted for 57% of total visits.
Figure 1.
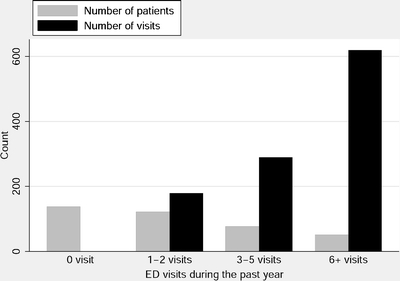
Number of patients and number of emergency department (ED) visits made by individuals according to 4 frequency levels of ED use during the past year. For example, on the far right side, 51 patients had 6 or more visits in the past year, accounting for a total of 620 ED visits.
Univariate analysis of factors associated with FEDV for COPD is shown in Table 1. Nonwhite race, uninsured patients, patients with Medicaid, and mixed disease patients were associated with an increased FEDV during the past year. Patients with an increased FEDV were less likely to be older, have a PCP, and were less likely to have completed high school education and have higher median household income. Sex was unrelated to FEDV. Patients with an increased FEDV were more likely to report a history of systemic steroid use, hospitalization for COPD, intubation for COPD, recent use of inhaled β-agonists and methylxanthine, and depression.
Multivariate analysis showed that Hispanic patients had two times increased incidence rate of ED visits in the past year compared with whites (Table 2). Several markers of COPD severity (e.g., history of hospitalization, intubation for COPD, and use of systemic corticosteroids and methylxanthine) were all independently associated with an increased FEDV. In the final multivariate model, having a PCP was the only factor for decreased FEDV. There were no significant two-way interactions in the final model.
Table 2.
Multivariate model of factors associated with frequency of emergency department visits for COPD exacerbation during the past year
| Variable | Incidence rate ratio | 95% Confidence interval |
|---|---|---|
| Age | 0.99 | 0.97–1.01 |
| Female sex | 0.86 | 0.66–1.13 |
| High school graduate | 0.81 | 0.61–1.07 |
| Race | ||
| White | 1 | Reference |
| Black | 1.23 | 0.88–1.74 |
| Hispanic | 1.97 | 1.16–3.33 |
| Others | 2.12 | 0.77–5.83 |
| Insurance status | ||
| Private | 1 | Reference |
| Medicaid | 1.41 | 0.89–2.24 |
| Other public | 0.96 | 0.68–1.35 |
| None | 1.64 | 0.98–2.73 |
| Has primary care provider | 0.51 | 0.31–0.82 |
| Prior hospitalization for COPD | 2.06 | 1.51–2.82 |
| Ever taken systemic corticosteroids for COPD | 1.57 | 1.16–2.13 |
| Ever intubated for COPD | 1.49 | 1.02–2.18 |
| Use of methylxanthine in past 4 weeks | 1.48 | 1.04–2.12 |
Chronic obstructive pulmonary disease (COPD)
In our cohort, 90% of patients reported having a PCP. Based on the formula, we estimated that approximately 100 ED visits (i.e., 10%) could have been prevented if all patients in our cohort had a PCP.
We repeated the multivariate model after excluding two patients reporting more than 30 visits, and the results slightly changed. History of intubation was no longer predictive of FEDV (IRR 1.30, 95% CI 0.90–1.87), whereas patients with Medicaid (IRR 1.61, 95% CI 1.03–2.53) and uninsured patients (IRR 2.03, 95% CI 1.22–3.36) became significantly associated with an increased FEDV compared with those with private insurance. The estimates of other predictors did not materially change. Excluding Canadian patients did not materially change the estimates of insurance status and the other variables in the final model (data not shown).
DISCUSSION
This large, prospective multicenter study is the first to examine factors associated with FEDV among COPD patients presenting to North American EDs for COPD exacerbation. Our results suggest that both disease and health care-related factors are associated with FEDV in this patient population. We believe that our findings may provide evidence as a basis for future development of efficacious interventions to reduce the substantial burden from frequent ED use for COPD care.
It is biologically plausible that patients with more severe COPD will require more ED visits compared to patients with mild disease. Evidence suggests that patients with severe COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 3) had an annual exacerbation frequency of 3.43 compared with 2.68 in those with moderate COPD (GOLD stage 2).21 Consistent with this finding, we further identified that frequent ED users are more likely to have several markers of severe COPD, including previous hospitalizations, prior intubations, and past use of systemic corticosteroids and methylxanthine. Similar to our findings, in a prospective study of asthma, previous hospitalization was the strongest predictor for frequent ED visits.22
To better understand other factors associated with FEDV in our study, it is important to distinguish recurrence of COPD exacerbation from relapse. The major difference is that the former refers to recurrent events over the course of a longer period of time, such as 1 year in our study. In contrast, relapse refers to revisit to the ED for the same exacerbation (usually within 2 to 4 weeks of the index ED visit). As a result, the predictors of relapse are not necessarily the predictors of FEDV in a longer period. Conceptually, relapse is more likely to be related to acute/medical factors, such as severity exacerbation (e.g., respiratory rate).23 By contrast, recurrent ED visits has a broader meaning, and therefore it may be connected to health care-related factors in addition to disease factors.
A number of studies have identified a set of social and health care-related predictors of FEDV across diseases, such as homelessness, poverty, socioeconomic distress, and government-supported insurance.24–27 In this study, we found two health care-related factors, insurance status (after excluding outliers) and lack of a PCP, that were associated with frequent ED use. Indeed, a high rate of ED visits may suggest that a population lacks access to preventive and routine care provided by PCP. As a result, when care is needed, patients sometimes resort to using the ED. This finding is consistent with a recent study that demonstrates an inverse association between primary care densities and ED visit densities in the United States.28 Our study further estimated that 1 in 10 ED visits could have been prevented if PCP is available for all patients. Therefore, primary care system may play a critical role in solving frequent ED utilization for COPD exacerbation.
Lack of a PCP is largely because of lack of health insurance.29 Therefore, uninsured COPD patients are also at greater risk of an increased FEDV in our study, compared to patients with any type of health insurance. This finding is consistent with the national trend that the continued increase in the number of uninsured parallels with the increase in the ED visits.30–33 In observational studies, an alternative explanation for the association between insurance and ED visits is confounding by indication. That is, patients who have insurance are more likely to be healthy than patient who do not have health insurance, and therefore they appeared to be less likely to visit the ED for COPD exacerbation.
Despite the enormous burden from ED visits, the recommendations regarding how to reduce ED visits in current COPD guidelines are still lacking.12,13 This is partly because of the lack of evidence. Many pharmacologic interventions have been associated with reductions in exacerbation; however, the drug effectiveness on decrease in ED visits remains unclear. More recent studies suggest that the improvement in exacerbation rate can be translated into reduced hospitalization rate,34,35 but no significant difference in ED visits.34 Another possible approach to reduce future ED visits is to provide PCP follow-up in COPD patients who are discharged from the ED. In a prospective study among patients who have been recently treated in the ED for COPD or asthma, follow-up office visits were associated with a significant reduction in the 90-day risk of an ED visit.36 Provision of post-ED PCP follow-up may fill in the gap between ED and primary care in the continuum of COPD care. In asthma, the current standard of practice for exacerbations includes a follow-up medical appointment within 3 to 5 days after ED visit.37 We believe that provision of PCP follow-up after ED visit may serve as a possible solution to reduce the burden of frequent ED visits in COPD patients.
There are relatively sparse data on racial/ethnic disparities in COPD. To our knowledge, this is the first study demonstrating racial/ethnic differences in ED utilization for COPD exacerbation. Similar to our findings, Boudreaux et al.38 have shown that black and Hispanic asthma patients were more likely to utilize the ED and concluded race/ethnicity-based deficiencies exist in asthma. In our study, after controlling for potential confounders such as educational level and insurance status, Hispanic patients remain more likely to utilize the ED, with a reduced IRR from 3.50 in univariate analysis to 1.97 in multivariate analysis. The persisting race/ethnicity differences in ED utilization can be explained by geographic access, transportation, primary language, health beliefs, patient perceptions of need, or patient–provider communication.39 The pathways through which racial/ethnicity affect health care utilization are complex. Further investigations are needed to better understand the underlying reasons for racial/ethnic differences in ED utilization in patients with COPD exacerbation.
Our results must be interpreted in the context of the study design. First, history of prior ED use was self-reported and there was no attempt to verify the accuracy of the stated information. However, as shown in the sensitivity analysis, our findings did not substantially change after excluding influential observations with high numbers of ED visits. Second, this study examined only patients who presented to the ED with an acute exacerbation, and thus it may not be generalizable to all stable, population-based COPD patients. However, because our goal is to characterize the frequent visitors to the ED, the findings are directly relevant to our objectives. Finally, because of the small sample size of Hispanic patients (n = 27, 7%), the racial/ethnic differences in ED utilization need to be replicated in future studies.
In summary, 13% of COPD patients had 6 or more ED visits in our cohort, accounting for 57% of the total ED visits for COPD exacerbation. Our results suggest that both disease and health care-related factors were associated with FEDV. Patients with markers of more severe COPD were associated with an increased FEDV, whereas patients who had a PCP were associated with a decreased FEDV. Multidisciplinary efforts through PCP follow-up should be assessed to test the effects on reducing the high morbidity and cost of recurrent COPD exacerbations. Hispanic patients were more likely to utilize the ED in our study. Further investigations are needed to better understand the underlying reasons for racial/ethnic differences in ED utilization in patients with COPD exacerbation.
Ackowledgments
The authors thank the EMNet investigators for their ongoing dedication to public health research, with an emphasis on the treatment and prevention of respiratory/allergy emergencies. The cohort studies were supported by an unrestricted grant from Boehringer Ingelheim (Ridgefield, Conn, USA and Burlington, Ontario, Canada).
EMNet Steering Committee: Michelle P. Blanda, MD; Edwin D. Boudreaux, PhD; Carlos A. Camargo, Jr., MD (Chair); Rita K. Cydulka, MD; Theodore J. Gaeta, DO, MPH; Susan Key, RN, MS, CEN; Steven Polevoi, MD; Michael S. Radeos, MD, MPH; and Benjamin C. Sun, MD, MPP.
EMNet Coordinating Center: Christina Ahn; Carlos A. Camargo, Jr., MD (Director); Sunday Clark, MPH, ScD; Lisa A. Dubois; Kate E. Delaney; Adit A. Ginde, MD; Andrea J. Pelletier, MS, MPH; Ashley F. Sullivan, MS, MPH; Chu-Lin Tsai, MD, MPH (all from the Massachusetts General Hospital, Boston, Mass, USA).
Principal Investigators at the 29 Participating Sites: FC Baker III (Maine Medical Center, Portland, Maine, USA); MP Blanda (Summa Health System, Akron, Ohio, USA); ED Boudreaux (Earl K. Long Memorial Hospital, Baton Rouge, La, USA); BE Brenner (The Brooklyn Hospital Center, Brooklyn, NY, USA); CA Camargo, Jr. (Massachusetts General Hospital, Boston, Mass, USA); RK Cydulka (MetroHealth Medical Center, Cleveland, Ohio, USA); TJ Gaeta (New York Methodist Hospital, Brooklyn, NY, USA); B Goldfeder (Shands Hospital at the Univeristy of Florida, Gainesville, Fla, USA); RJ Grant (Hartford Hospital, Hartford, Conn, USA); RO Gray (Hennepin County Medical Center, Minneapolis, Minn, USA); A Guttman (Sir Mortimer B. Davis, Jewish General Hospital, Montreal, Quebec, Canada); LW Kreplick (Christ Hospital and Medical Center, Oak Lawn, Ill, USA); DS Mackey (Lethbridge Regional Hospital, Lethbridge, Alberta, Canada); A Mangione (Albert Einstein Medical Center, Philadelphia, Pa, USA); J Peters (University of Texas Health Sciences Center at San Antonio, San Antonio, Tex, USA); MS Radeos (Lincoln Medical Center, Bronx, NY); PL Rice (Brigham and Women’s Hospital, Boston, Mass, USA); BH Rowe (University of Alberta Hospital, Edmonton, Alberta, Canada); M Sama (St. Joseph Mercy Hospital, Ann Arbor, Mich, USA); D Schreiber (Stanford University Medical Center, Stanford, Calif, USA); NI Shapiro (Beth Israel Deaconess Medical Center, Boston, Mass, USA); PC Shukla (University of Texas Southwestern Medical Center, Dallas, Tex, USA); D Sinclair (Queen Elizabeth II Health Science Centre, Halifax, Nova Scotia, Canada); H Smithline (Baystate Medical Center, Springfield, Mass, USA); PE Sokolove (UC Davis Medical Center, Sacramento, Calif, USA); M Steffens (Palmetto Richland Memorial Hospital, Columbia, SC, USA); CA Terregino (Cooper Hospital/University Medical Center, Camden, NJ, USA); A Travers (Royal Alexandria Hospital, Edmonton, Alberta, Canada); and EJ Weber (UCSF Medical Center, San Francisco, Calif, USA).
Conflict of interest statement Dr. Camargo has received financial support (research grants, consulting, lectures) from AstraZeneca (Wilmington, Del, USA), Boehringer Ingelheim (Ridgefield, Conn, USA), GlaxoSmithKline (Research Triangle Park, NC, USA), Novartis (East Hanover, NJ, USA), and Schering Plough (Kenilworth, NJ, USA). All other authors declare that they have no conflicts of interest.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- ED
Emergency department
References
- 1.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ 2002;51:1–16. [PubMed]
- 2.Urbano FL, Pascual RM. Contemporary issues in the care of patients with chronic obstructive pulmonary disease. J Manag Care Pharm 2005;11:S2–13; quiz S14–6. [DOI] [PMC free article] [PubMed]
- 3.Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics 2005;23:619–37. [DOI] [PubMed]
- 4.Chapman KR, Bourbeau J, Rance L. The burden of COPD in Canada: results from the Confronting COPD survey. Respir Med 2003;97(suppl C):S23–31. [DOI] [PubMed]
- 5.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–22. [DOI] [PubMed]
- 6.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–52. [DOI] [PMC free article] [PubMed]
- 7.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925–31. [DOI] [PMC free article] [PubMed]
- 8.Tsai CL, Clark S, Cydulka RK, Rowe BH, Camargo CA Jr. Factors associated with hospital admission among emergency department patients with chronic obstructive pulmonary disease exacerbation. Acad Emerg Med 2007;14:6–14. [DOI] [PubMed]
- 9.Garcia-Aymerich J, Monso E, Marrades RM, et al. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J Respir Crit Care Med 2001;164:1002–7. [DOI] [PubMed]
- 10.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:158–64. [DOI] [PubMed]
- 11.Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA Jr. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: the Multicenter Airway Research Collaboration. J Am Geriatr Soc 2003;51:908–16. [DOI] [PubMed]
- 12.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;152:S77–121. [PubMed]
- 13.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001;163:1256–76. [DOI] [PubMed]
- 14.Radeos MS, Cydulka RK, Rowe BH, Barr RG, Camargo CA Jr. Validation of clinical diagnosis of COPD among patients presenting to the emergency department. Am J Respir Crit Care Med 2001;163:A912.
- 15.CACI. The sourcebook of zip code demographics. Fairfax, Va: CACI; 2002.
- 16.Canada H. Federal census data: Statistics Canada. Ottawa, Ontario, Canada: Health Canada; 1996.
- 17.Glynn RJ, Buring JE. Ways of measuring rates of recurrent events. BMJ 1996;312:364–7. [DOI] [PMC free article] [PubMed]
- 18.Tsai CL. Appropriate statistical treatment of frequent emergency department visits in health services research. Ann Emerg Med 2007;49:385. [DOI] [PubMed]
- 19.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989;79:340–9. [DOI] [PMC free article] [PubMed]
- 20.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 1974;99:325–32. [DOI] [PubMed]
- 21.Donaldson GC, Seemungal TA, Patel IS, Lloyd-Owen SJ, Wilkinson TM, Wedzicha JA. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J 2003;22:931–6. [DOI] [PubMed]
- 22.Pesola GR, Xu F, Ahsan H, Sternfels P, Meyer IH, Ford JG. Predicting asthma morbidity in Harlem emergency department patients. Acad Emerg Med 2004;11:944–50. [DOI] [PubMed]
- 23.Kim S, Emerman CL, Cydulka RK, Rowe BH, Clark S, Camargo CA Jr. Prospective multicenter study of relapse following emergency department treatment of COPD exacerbation. Chest 2004;125:473–81. [DOI] [PubMed]
- 24.Mandelberg JH, Kuhn RE, Kohn MA. Epidemiologic analysis of an urban, public emergency department’s frequent users. Acad Emerg Med 2000;7:637–46. [DOI] [PubMed]
- 25.McCusker J, Cardin S, Bellavance F, Belzile E. Return to the emergency department among elders: patterns and predictors. Acad Emerg Med 2000;7:249–59. [DOI] [PubMed]
- 26.Purdie FR, Honigman B, Rosen P. The chronic emergency department patient. Ann Emerg Med 1981;10:298–301. [DOI] [PubMed]
- 27.Sun BC, Burstin HR, Brennan TA. Predictors and outcomes of frequent emergency department users. Acad Emerg Med 2003;10:320–8. [DOI] [PubMed]
- 28.Richman IB, Clark S, Sullivan AF, Camargo CA Jr. National Study of the Relation of Primary Care Shortages to Emergency Department Utilization. Acad Emerg Med. 2007;14:279–82. [DOI] [PubMed]
- 29.Institute of Medicine (U.S.). Committee on the Consequences of Uninsurance. Coverage matters: insurance and health care. Washington, DC: National Academy Press; 2001.
- 30.National Center for Health Statistics (U.S.). National Hospital Ambulatory Medical Care Survey. Emergency department summary. Hyattsville, Md: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2001.
- 31.National Center for Health Statistics (U.S.). National Hospital Ambulatory Medical Care Survey. Emergency department summary. Hyattsville, Md: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2004.
- 32.Bureau of the Census (U.S.). Health insurance coverage. Washington, DC: Bureau of the Census, United States; 2001.
- 33.Bureau of the Census (U.S.). Health insurance coverage. Washington, DC: Bureau of the Census, United States; 2005.
- 34.Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. [DOI] [PubMed]
- 35.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med 2005;143:317–26. [DOI] [PubMed]
- 36.Sin DD, Bell NR, Svenson LW, Man SF. The impact of follow-up physician visits on emergency readmissions for patients with asthma and chronic obstructive pulmonary disease: a population-based study. Am J Med 2002;112:120–5. [DOI] [PubMed]
- 37.National Asthma Education and Prevention Program. Expert panel report II: Guidelines for the diagnosis and management of asthma. Bethesda, Md: National Institutes of Health; 1997.
- 38.Boudreaux ED, Emond SD, Clark S, Camargo CA Jr. Acute asthma among adults presenting to the emergency department: the role of race/ethnicity and socioeconomic status. Chest 2003;124:803–12. [DOI] [PubMed]
- 39.Baker DW, Stevens CD, Brook RH. Determinants of emergency department use: are race and ethnicity important? Ann Emerg Med 1996;28:677–82. [DOI] [PubMed]


