Table 1.
Chemical structures of the phenolic compounds used in the present study.
| Class | Derivatives | Substituents | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 * | 3 | 5 | 7 | 3′ | 4′ | 5′ | |||
| Flavones | Acacetin | H | OH | OH | H | OCH3 | H | ||
| Apigenin | H | OH | OH | H | OH | H | |||
| Flavone | H | H | H | H | H | H | |||
| Luteolin | H | OH | OH | OH | OH | H | |||
| Lut-7-glucoside | H | OH | OGlc | OH | OH | H | |||
| Vitexin | H | OH | OH | H | OH | H | 8 Glc | ||
|
| |||||||||
| Isoflavones | Daidzein | H | H | OH | H | OH | H | ||
| Genistein | H | OH | OH | H | OH | H | |||
| Genistin | H | OH | OGlc | H | OH | H | |||
|
| |||||||||
| Flavonols | Isorhamnetin | OH | OH | OH | OCH3 | OH | H | ||
| Kaempferol | OH | OH | OH | H | OH | H | |||
| Morin | OH | OH | OH | H | OH | H | 2′ OH | ||
| Myricetin | OH | OH | OH | OH | OH | OH | |||
| Quercetin | OH | OH | OH | OH | OH | H | |||
| Quercitrin | ORha | OH | OH | OH | OH | H | |||
| Rhamnetin | OH | OH | OCH3 | OH | OH | H | |||
| Rutin | ORu | OH | OH | OH | OH | H | |||
|
| |||||||||
| Flavanones | Naringenin | H | OH | OH | H | OH | H | ||
| Naringin | H | OH | ONeo | H | OH | H | |||
| Taxifolin | OH | OH | OH | OH | OH | H | |||
|
| |||||||||
| Flavan-3-ols | +Catechin | OH
|
OH | OH | OH | OH | H | ||
| −Epicatechin | OH
|
OH | OH | OH | OH | H | |||
| Procyanidin B1 | Dimer of epicatechin and catechin linked via their carbons 4 and 8, respectively. | ||||||||
| Procyanidin B2 | Dimer of two epicatechin molecules linked via carbons 4 and 8. | ||||||||
|
| |||||||||
| Anthocyanins | Cyanidin | OH | OH | OH | OH | OH | H | ||
| Pelargonidin | OH | OH | OH | H | OH | H | |||
|
| |||||||||
| HBA | Benzoic acid | H | H | 4 H | |||||
| Dodecyl gallate | COO(CH2)11CH3 | OH | OH | ||||||
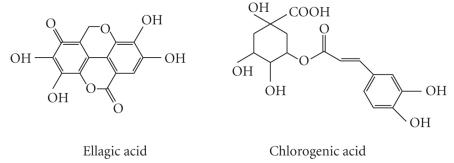
| Ellagic acid | see below | ||||||||
| Gallic acid | OH | OH | |||||||
| Methyl gallate | COOCH3 | OH | OH | ||||||
| Octyl gallate | COOCH2(CH2)6CH3 | OH | OH | ||||||
| Syringic acid | OCH3 | OCH3 | |||||||
|
| |||||||||
| HCA | Chlorogenic acid | see below | |||||||
| Ferulic acid | OCH3 | ||||||||
| Sinapic acid | OCH3 | OCH3 | |||||||
*if other than in basic structure
Glc = glycoside, Rha = rhamnoside, Ru = rutinoside, Neo = neohesperidoside
 OH group is in front of plane of paper,
OH group is in front of plane of paper,  OH group is behind plane of paper
OH group is behind plane of paper