Fig 2. Direct inhibition of CDK9 activity with HA-dnCDK9 in 293T cells inhibits RNAPII phosphorylation on CTD Ser-2 and HIV-1 transcription, but fails to affect the overall rates of cellular transcription and cell proliferation and viability.
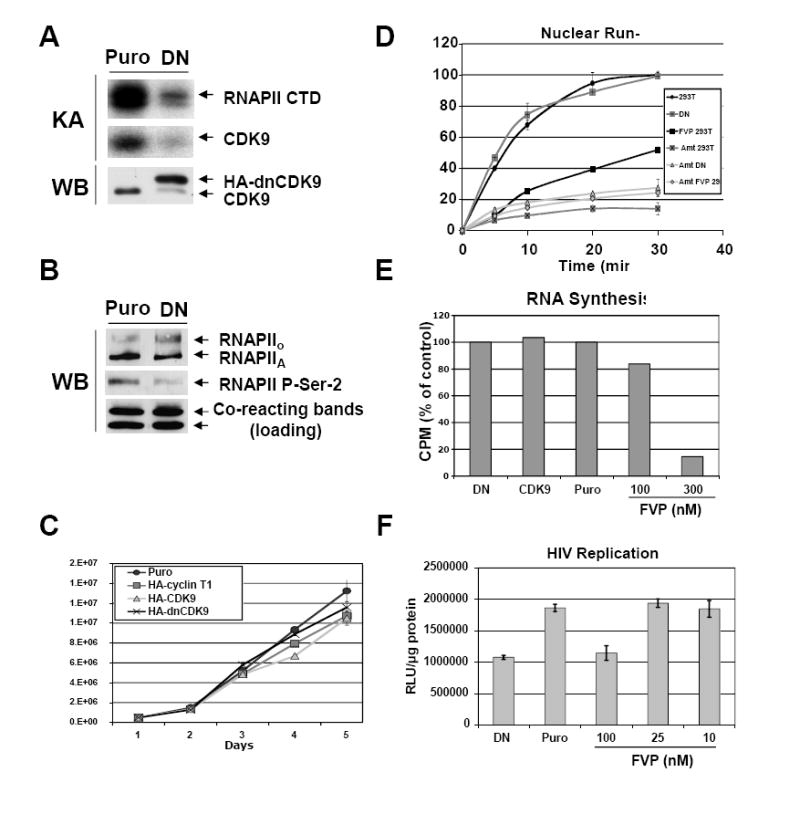
293 cells stably expressing HA-dnCDK9, HA-CDK9 or HA-cyclin T1 and control puromycin resistant (Puro) cells were generated by lentiviral infection followed by selection with puromycin. Lentiviruses were generated as described in Fig 1A and the Methods section using the corresponding pCPP transfer construct for each transgene. Clones expressing the highest transgene levels were selected for further experiments. (A) Ectopic expression of dnCDK9 inhibits CDK9-associated kinase activity. HA-dnCDK9 and endogenous CDK9 expression was determined by Western blot analysis of whole cell lysates of control (Puro) and HA-dnCDK9 (DN) expressing cells (WB). Cellular CDK9 was immunoprecipitated with anti-CDK9 antibodies. Kinase reactions were performed by incubating the immunoprecipitates with GST-RNAPII-CTD (exogenous substrate) as described in the Materials and Methods section. RNAPII-CTD phosphorylation and CDK9 autophosphorylation is shown (KA). (B) Ectopic expression of HA-dnCDK9 inhibits phosphorylation of RNAPII on Ser-2 in vivo. The effects of HA-dnCDK9 expression on RNAPII Ser-2 phosphorylation were determined by Western blot analysis. The expression of total RNAPII is also shown. Cross-reacting bands detected with the anti-Ser-2 antibody are shown as loading control. (C) Cell proliferation and viability of exponentially growing cells was measured by trypan blue staining and cell counting. No significant increase in cell death was detected in cells expressing HA-dnCDK9, HA-CDK9 or HA-cyclin T1. (D) The rates of cellular transcription were determined via run-on assays in 293T and HA-dnCDK9 (DN) cells as described in the Methods section. The effects of FVP (300 nM) and/or α-amanitin (Amt) were also determined where indicated. (E) RNA synthesis in HA-dnCDK9 (DN), HA-CDK9, Puro cells and Puro cells treated with the indicated concentrations of FVP was determined as by measuring [3H]-uridine incorporation into cellular RNA as described in the Methods section. (F) Single-round HIV-1 transcription assays were performed by infecting HA-dnCDK9 (DN), Puro and FVP treated Puro cells with HIV-1-luc viruses pseudotyped with a VSV-G envelope. HIV-1 transcription was measured by determining luciferase activity 48 h post infection in duplicate samples (see Methods section).
