Abstract
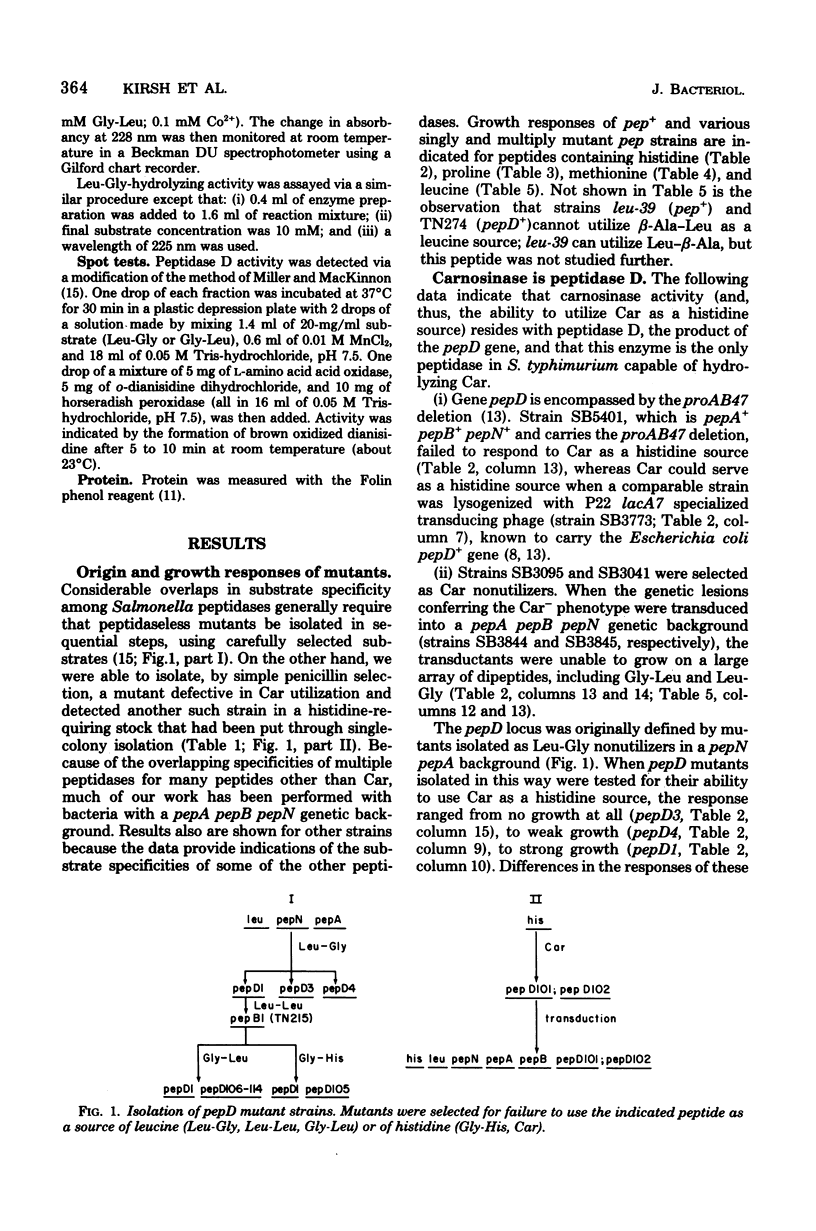
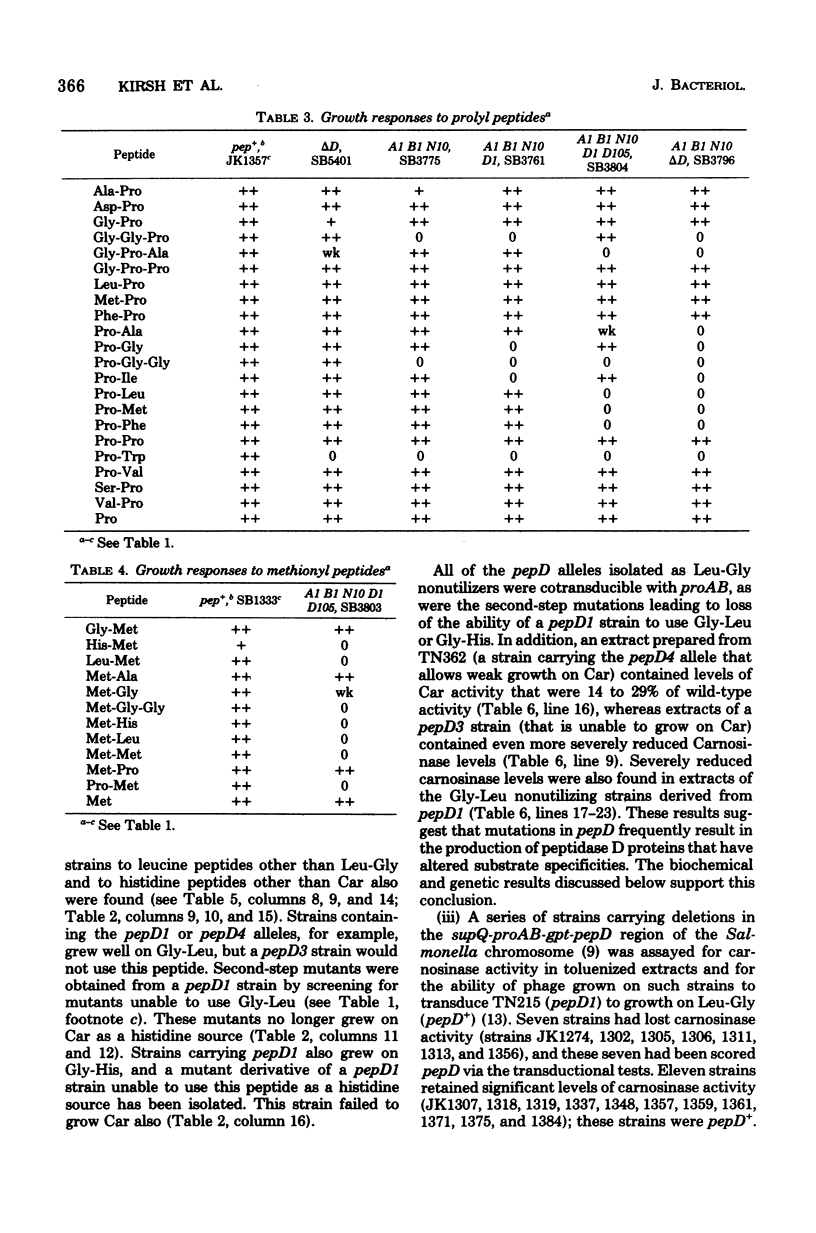
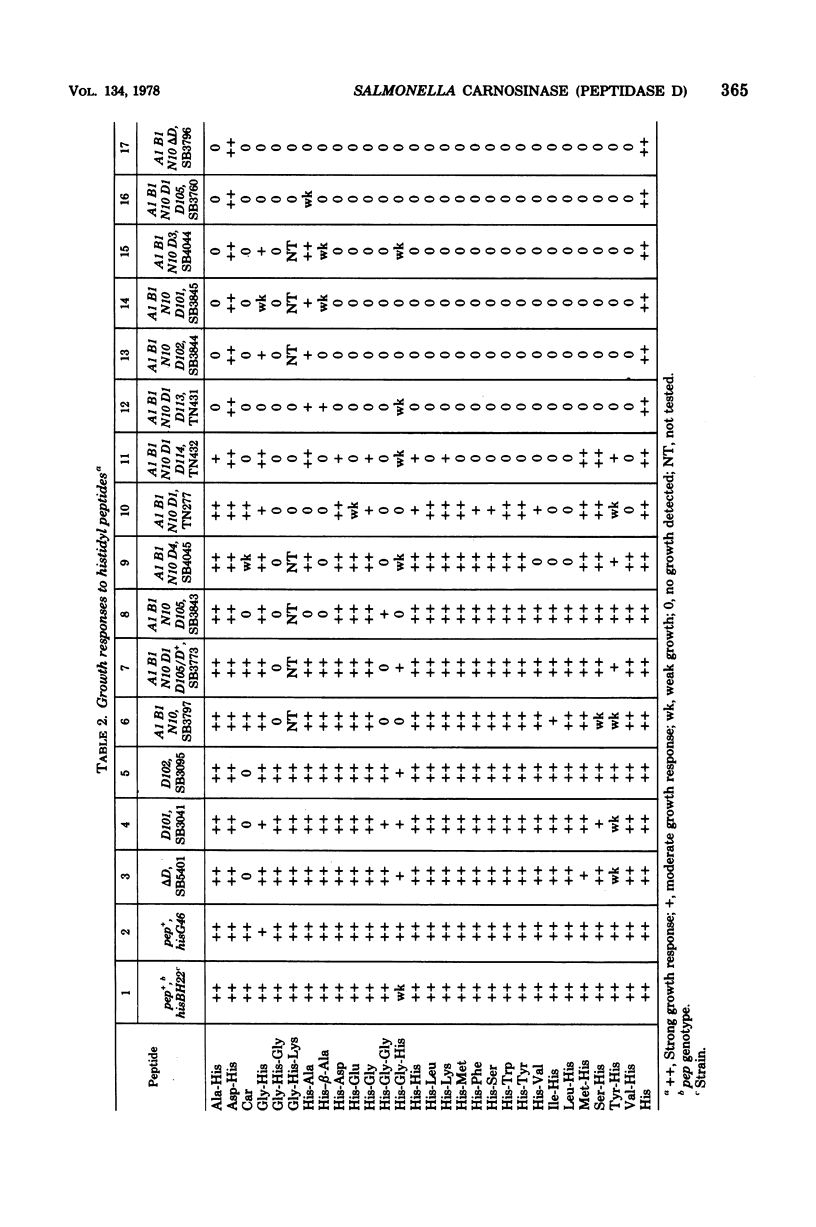
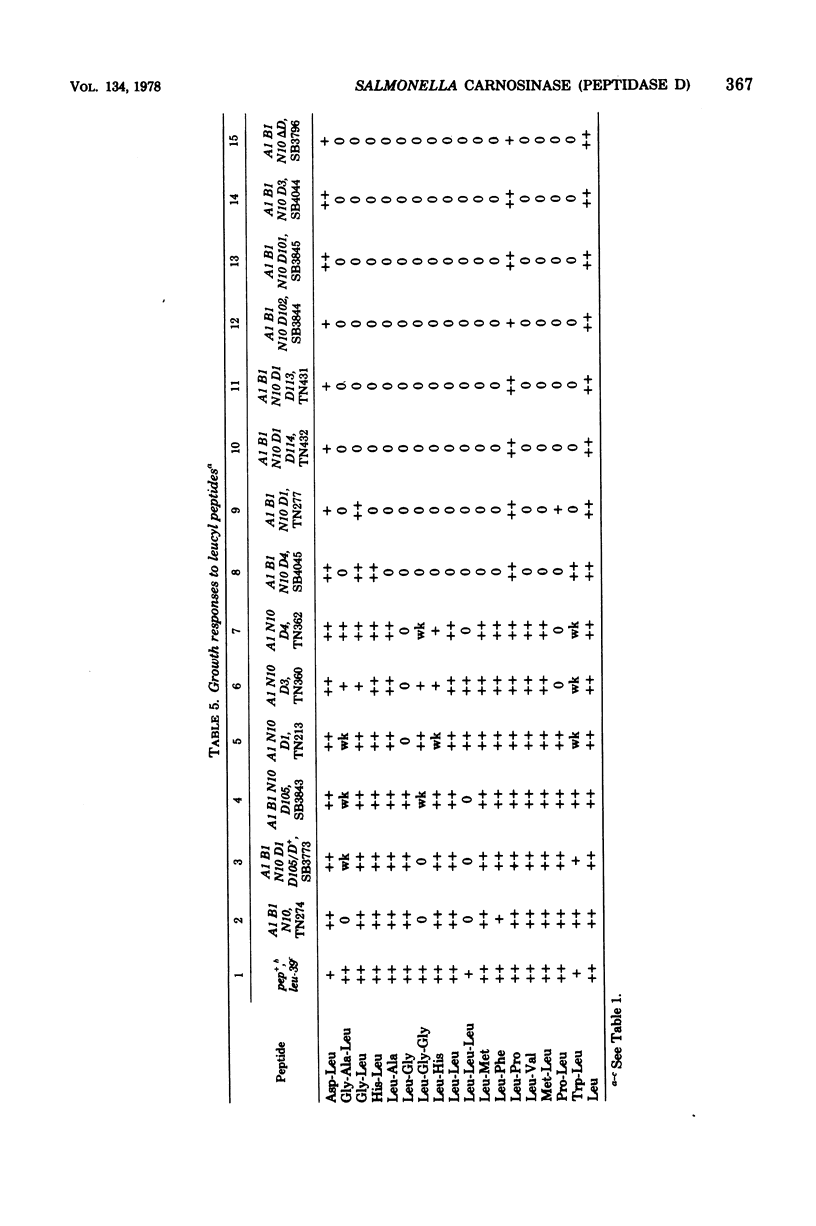
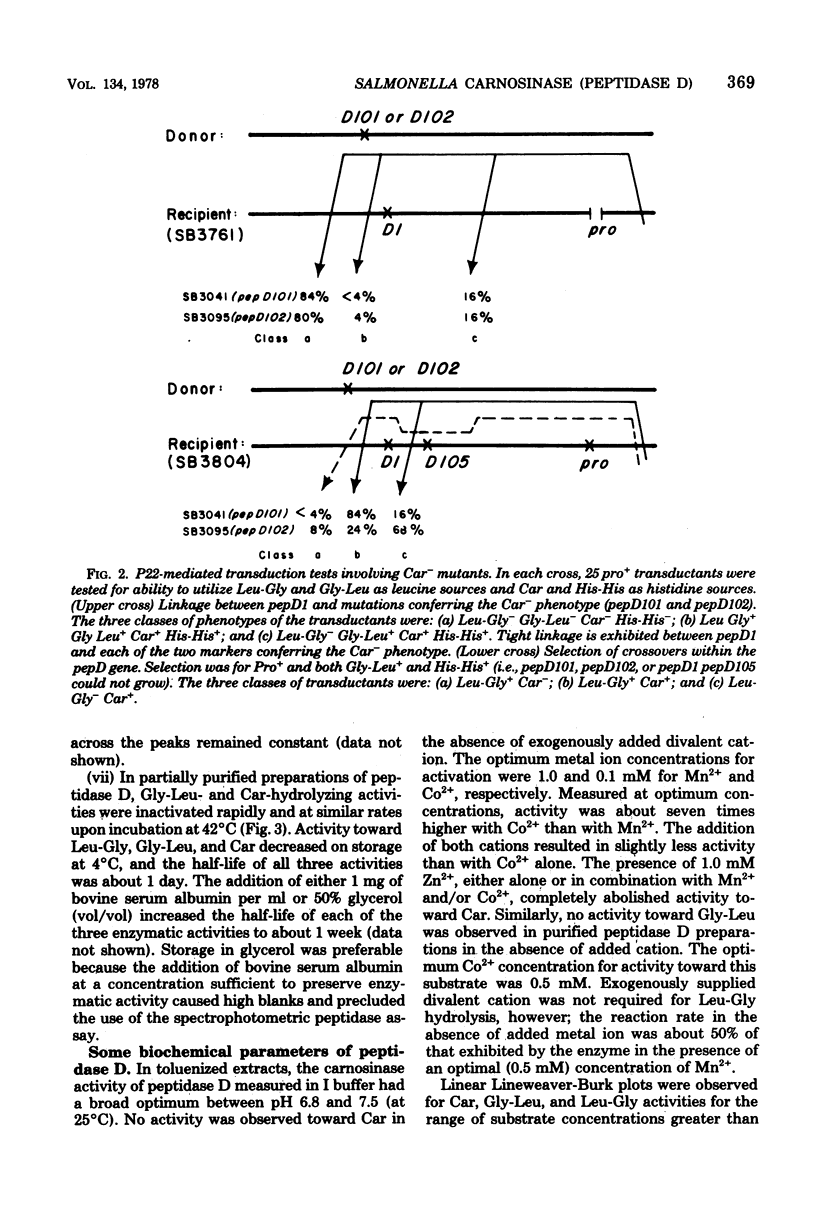
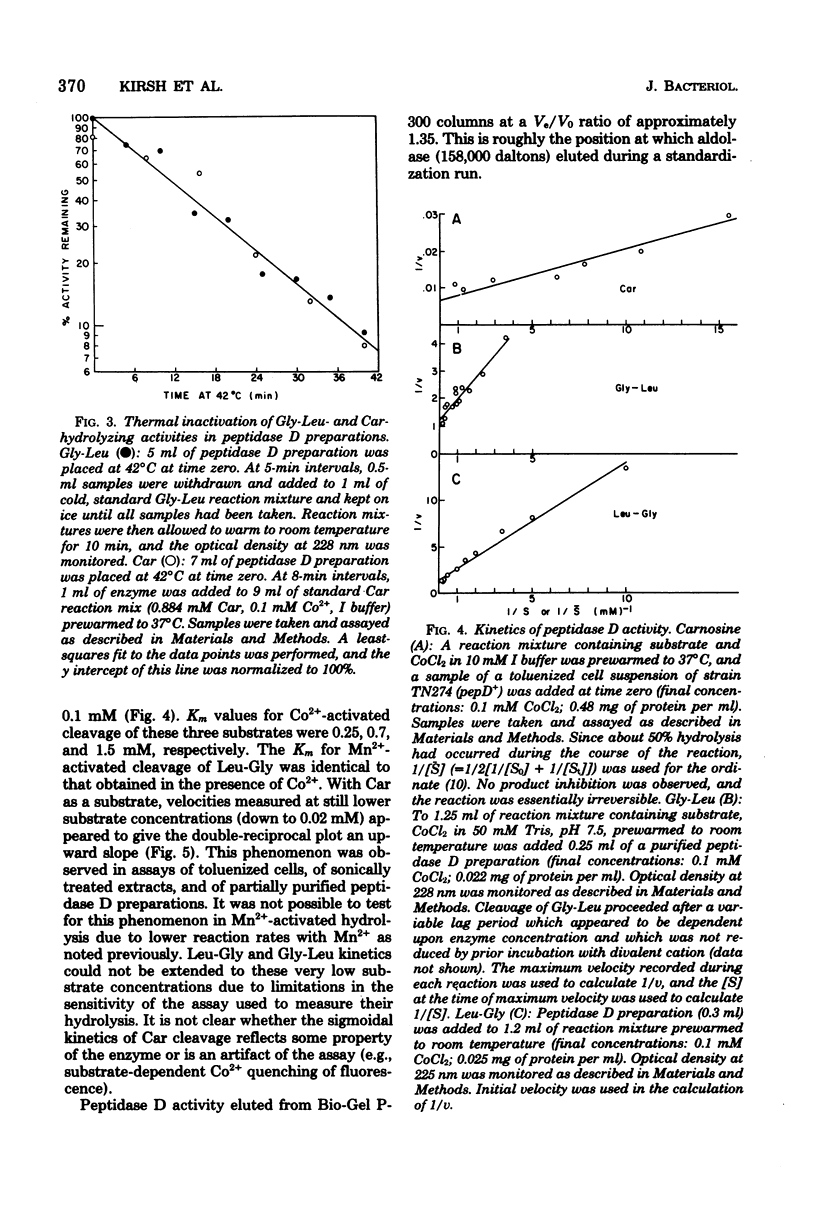
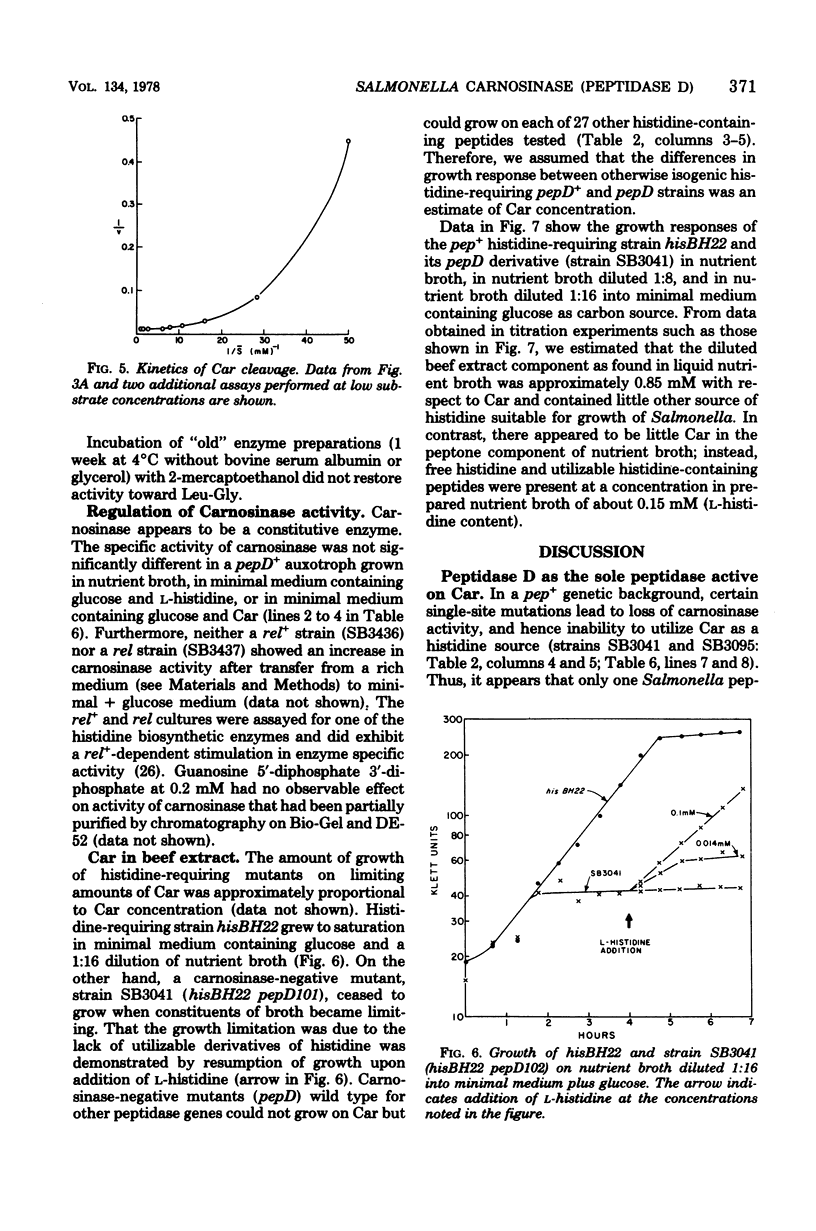
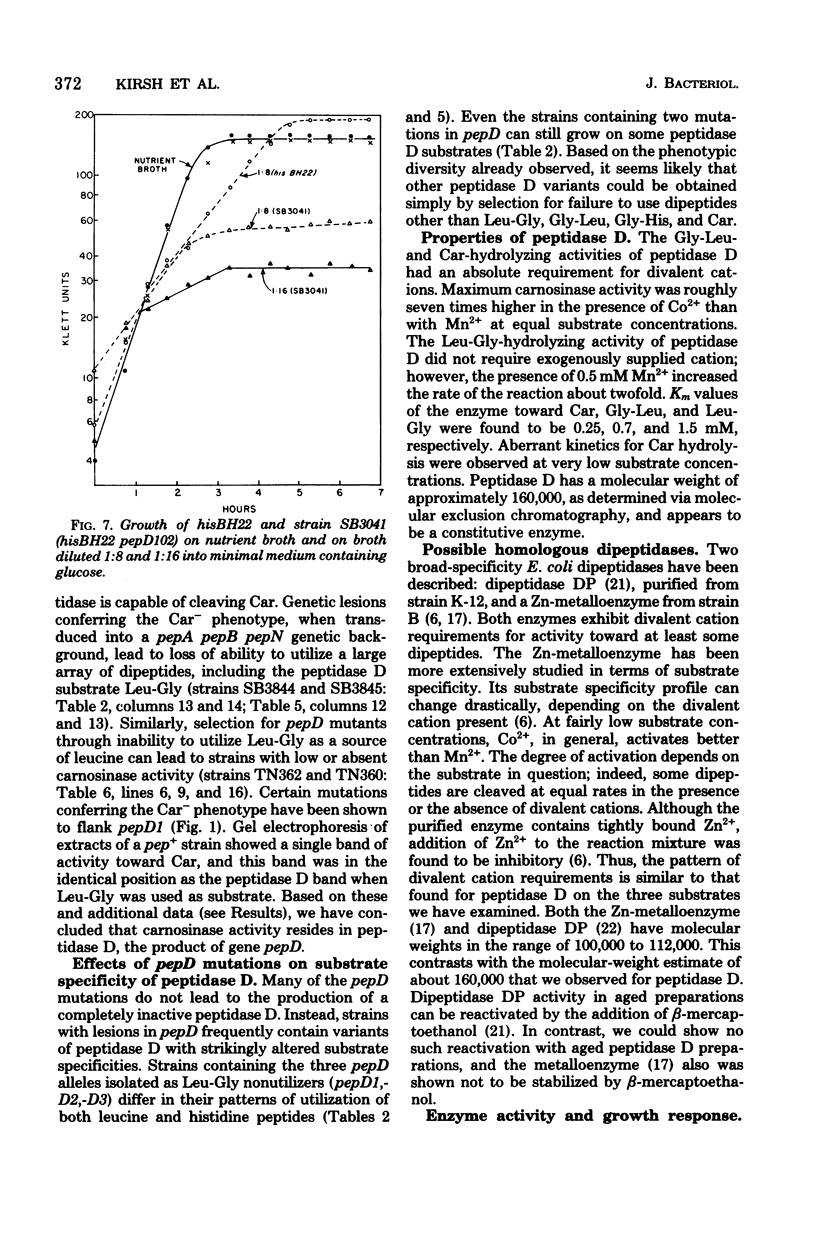
Wild-type Salmonella typhimurium can use carnosine (beta-alanyl-L-histidine) as a source of histidine, but carnosine utilization is blocked in particular mutants defective in the constitutive enzyme peptidase D, the product of the pepD gene. Biochemical evidence for assigning carnosinase activity to peptidase D (a broad-specificity dipeptidase) includes: (i) coelution of carnosinase and dipeptidase activity from diethylaminoethyl-cellulose and Bio-Gel P-300 columns; (ii) coelectrophoresis of carnosinase and dipeptidase on polyacrylamide gels; and (iii) inactivation of carnosinase and dipeptidase activities at identical rates at both 4 and 42 degrees C. Genetic evidence indicates that mutations leading to loss of carnosinase activity map at pepD. Several independent pepD mutants have been isolated by different selection procedures, and the patterns of peptide utilization of strains carrying various pepD alleles have been studied. Many pepD mutations lead to the production of partially active peptidase D enzymes with substrate specificities that differ strikingly from those of the wild-type enzyme. The growth yields of carnosinase-deficient strains growing in Difco nutrient broth indicate that carnosine is the major utilizable source of histidine in this medium.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Shumas S. R. Genetic separation of hypoxanthine and guanine-xanthine phosphoribosyltransferase activities by deletion mutations in Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):910–916. doi: 10.1128/jb.112.2.910-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman S., Gatmaitan J. S., Patterson E. K. The relationship of extrinsic and intrinsic metal ions to the specificity of a dipeptidase from Escherichia coli B. Biochemistry. 1974 Oct 22;13(22):4486–4494. doi: 10.1021/bi00719a003. [DOI] [PubMed] [Google Scholar]
- Holzer H., Betz H., Ebner E. Intracellular proteinases in microorganisms. Curr Top Cell Regul. 1975;9:103–156. doi: 10.1016/b978-0-12-152809-6.50011-1. [DOI] [PubMed] [Google Scholar]
- Hoppe I., Roth J. Specialized transducing phages derived from salmonella phage P22. Genetics. 1974 Apr;76(4):633–654. doi: 10.1093/genetics/76.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper J. Evolution of a new gene substituting for the leuD gene of Salmonella typhimurium: characterization of supQ mutations. J Bacteriol. 1974 Sep;119(3):937–951. doi: 10.1128/jb.119.3.937-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee H. J., Wilson I. B. Enzymic parameters: measurement of V and Km. Biochim Biophys Acta. 1971 Sep 22;242(3):519–522. doi: 10.1016/0005-2744(71)90144-6. [DOI] [PubMed] [Google Scholar]
- McHugh G. L., Miller C. G. Isolation and characterization of proline peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):364–371. doi: 10.1128/jb.120.1.364-371.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G. Gentic mapping of Salmonella typhimurium peptidase mutations. J Bacteriol. 1975 Apr;122(1):171–176. doi: 10.1128/jb.122.1.171-176.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. Annu Rev Microbiol. 1975;29:485–504. doi: 10.1146/annurev.mi.29.100175.002413. [DOI] [PubMed] [Google Scholar]
- Patterson E. K., Gatmaitan J. S., Hayman S. Substrate specificity and pH dependence of dipeptidases purified from Escherichia coli B and from mouse ascites tumor cells. Biochemistry. 1973 Sep 11;12(19):3701–3709. doi: 10.1021/bi00743a020. [DOI] [PubMed] [Google Scholar]
- Payne J. W. Variations in the peptidase activities of Escherichia coli in response to environmental changes. J Gen Microbiol. 1972 Jul;71(2):281–291. doi: 10.1099/00221287-71-2-281. [DOI] [PubMed] [Google Scholar]
- Simmonds S., Szeto K. S., Fletterick C. G. Soluble tri- and dipeptidases in Escherichia coli K-12+. Biochemistry. 1976 Jan 27;15(2):261–271. doi: 10.1021/bi00647a004. [DOI] [PubMed] [Google Scholar]
- Sussman A. J., Gilvarg C. Peptidases in Escherichia coli K-12 capable of cleaving lysine homopeptides. J Biol Chem. 1970 Dec 25;245(24):6518–6524. [PubMed] [Google Scholar]
- Taylor S., Tappel A. L. Lysosomal peptidase measurement by sensitive fluorometric amino acid analysis. Anal Biochem. 1973 Nov;56(1):140–148. doi: 10.1016/0003-2697(73)90178-4. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Winkler M. E., Roth D. J., Hartman P. E. Promoter- and attenuator-related metabolic regulation of the Salmonella typhimurium histidine operon. J Bacteriol. 1978 Feb;133(2):830–843. doi: 10.1128/jb.133.2.830-843.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


