Abstract
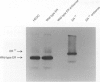
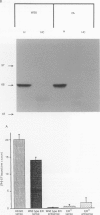
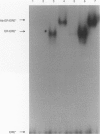
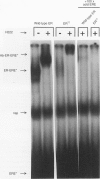
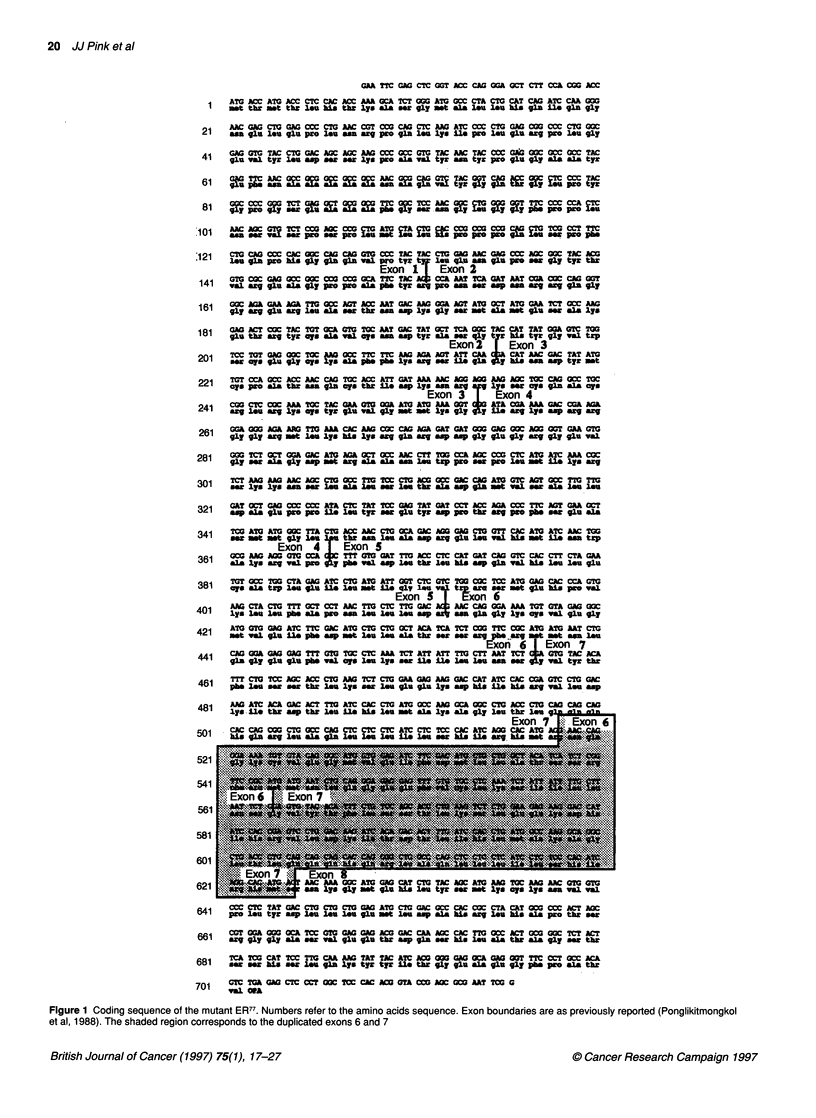
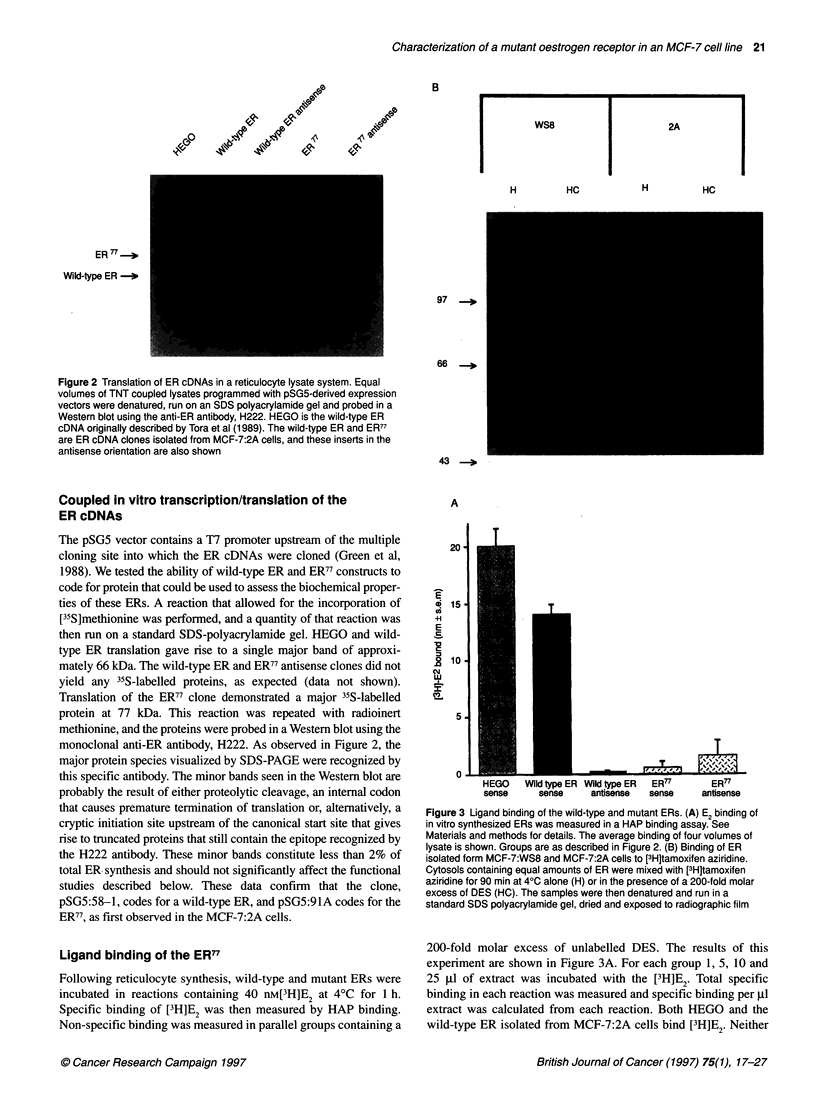
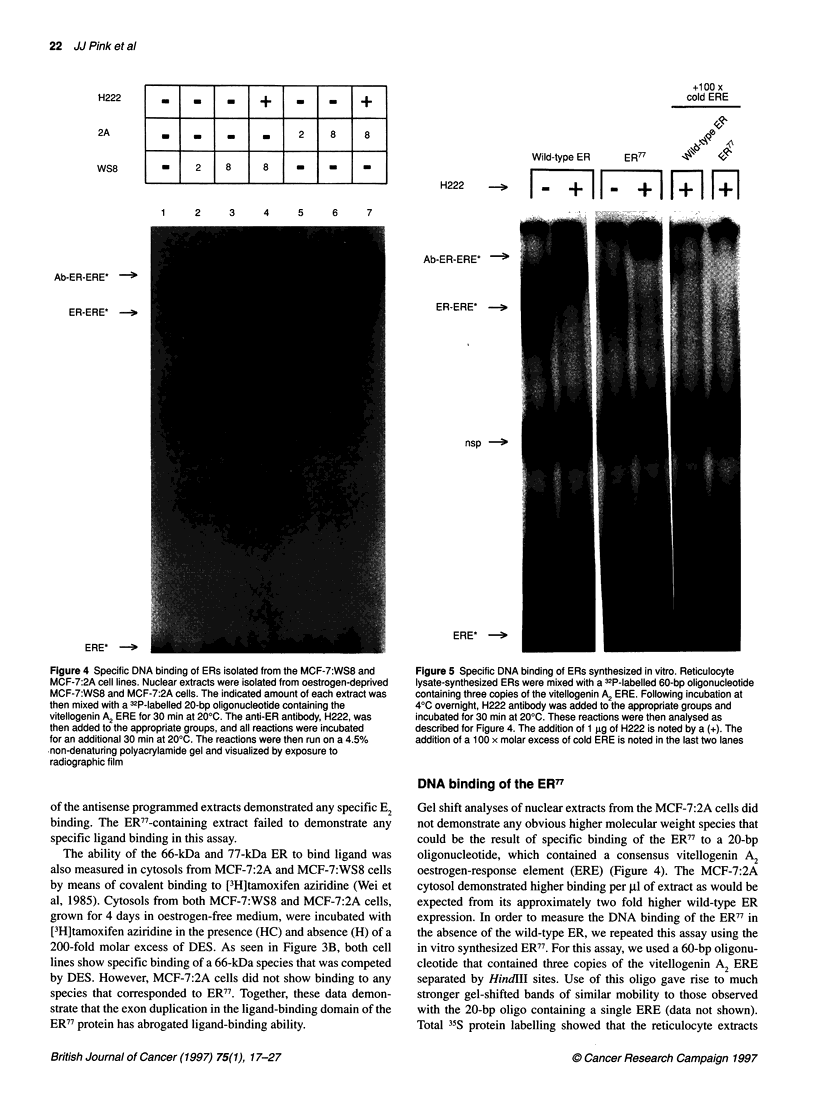
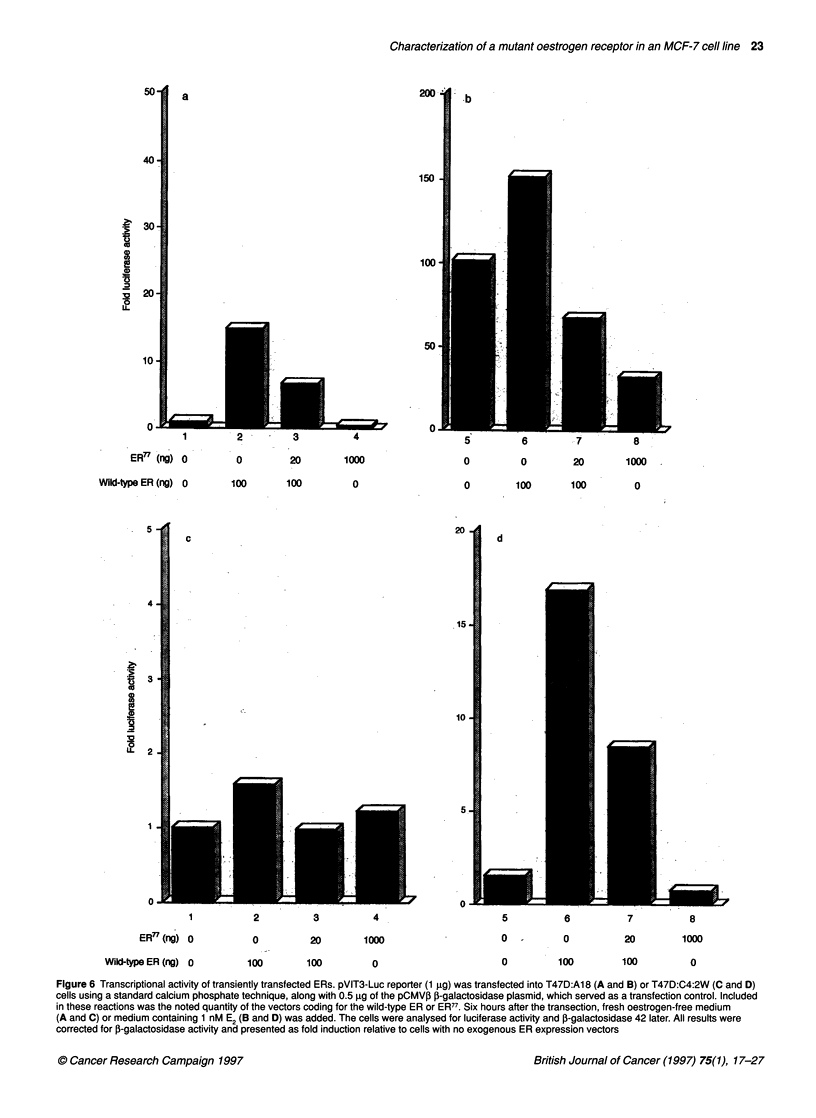
We have cloned and characterized a 77-kDa oestrogen receptor (ER) from an oestrogen-independent subclone of the MCF-7 human breast cancer cell line. This receptor contains an in-frame, tandem duplication of exons 6 and 7, located in the steroid-binding domain of the ER. This mutation has abrogated ligand binding, but not DNA binding, in this mutant ER. We previously described the partial structure of a unique oestrogen receptor (ER) that is expressed in an oestrogen-independent MCF-7:2A subclone of the breast cancer cell line MCF-7 (Pink JJ, Wu SQ, Wolf DM, Bilimoria MM, Jordan VC 1996a, Nucleic Acids Res 24 962-969). Sequence analyses determined the molecular weight of this 80-kDa ER to be 77 kDa, and hereafter this protein will be designated as ER77. Examination of the entire coding sequence of the ER77 mRNA indicates that it contains a tandem duplication of exons 6 and 7. Using a coupled transcription/translation system, a 77-kDa ER, which corresponds to the protein observed in the MCF-7:2A cells, was expressed. The ER77 protein does not bind the ligands [3H] oestradiol or [3H]tamoxifen aziridine. In DNA binding gel shift assays, the in vitro synthesized ER77 binds to a consensus vitellogenin A2 oestrogen-response element. In transient transfection experiments, the mutant ER, alone or in combination with the wild-type ER, does not induce expression of an oestrogen-responsive luciferase reporter construct. In fact, expression of the ER77 in the ER-positive T47D:A18 cell line inhibits E2-induced luciferase expression. Overexpression of wild-type ER in T47D:A18 cells leads to elevated constitutive expression of the luciferase reporter, which was inhibited by co-transfection with ER77. These data suggest that the ER77 can interfere with normal ER activity and does not act as a constitutive activator of oestrogen-independent growth in MCF-7:2A cells. Consequently, the constitutive growth observed in MCF-7:2A cells is probably the result of other ER-mediated pathways.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. C., Locke E. R., Soule H. D. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973 Sep 10;248(17):6251–6253. [PubMed] [Google Scholar]
- Cailleau R., Young R., Olivé M., Reeves W. J., Jr Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974 Sep;53(3):661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catherino W. H., Jordan V. C. Increasing the number of tandem estrogen response elements increases the estrogenic activity of a tamoxifen analogue. Cancer Lett. 1995 May 25;92(1):39–47. doi: 10.1016/0304-3835(95)03755-l. [DOI] [PubMed] [Google Scholar]
- Cho H., Katzenellenbogen B. S. Synergistic activation of estrogen receptor-mediated transcription by estradiol and protein kinase activators. Mol Endocrinol. 1993 Mar;7(3):441–452. doi: 10.1210/mend.7.3.7683375. [DOI] [PubMed] [Google Scholar]
- Fritsch M., Anderson I., Gorski J. Structural characterization of the trypsinized estrogen receptor. Biochemistry. 1993 Dec 21;32(50):14000–14008. doi: 10.1021/bi00213a033. [DOI] [PubMed] [Google Scholar]
- Fritsch M., Leary C. M., Furlow J. D., Ahrens H., Schuh T. J., Mueller G. C., Gorski J. A ligand-induced conformational change in the estrogen receptor is localized in the steroid binding domain. Biochemistry. 1992 Jun 16;31(23):5303–5311. doi: 10.1021/bi00138a009. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G. L., Fitch F. W., Jensen E. V. Monoclonal antibodies to estrophilin: probes for the study of estrogen receptors. Proc Natl Acad Sci U S A. 1980 Jan;77(1):157–161. doi: 10.1073/pnas.77.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince B. A., Zhuang Y., Wrenn C. K., Shapiro D. J., Katzenellenbogen B. S. Powerful dominant negative mutants of the human estrogen receptor. J Biol Chem. 1993 Jul 5;268(19):14026–14032. [PubMed] [Google Scholar]
- Jiang S. Y., Langan-Fahey S. M., Stella A. L., McCague R., Jordan V. C. Point mutation of estrogen receptor (ER) in the ligand-binding domain changes the pharmacology of antiestrogens in ER-negative breast cancer cells stably expressing complementary DNAs for ER. Mol Endocrinol. 1992 Dec;6(12):2167–2174. doi: 10.1210/mend.6.12.1491696. [DOI] [PubMed] [Google Scholar]
- Jiang S. Y., Wolf D. M., Yingling J. M., Chang C., Jordan V. C. An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Mol Cell Endocrinol. 1992 Dec;90(1):77–86. doi: 10.1016/0303-7207(92)90104-e. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Kendra K. L., Norman M. J., Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987 Aug 15;47(16):4355–4360. [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Luyten G. P., Hoogeveen A. T., Galjaard H. A fluorescence staining method for the demonstration and measurement of lysosomal enzyme activities in single cells. J Histochem Cytochem. 1985 Sep;33(9):965–968. doi: 10.1177/33.9.3926869. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. S., Meisner L. F., Wu S. Q., Jordan V. C. Short- and long-term estrogen deprivation of T47D human breast cancer cells in culture. Eur J Cancer Clin Oncol. 1989 Dec;25(12):1777–1788. doi: 10.1016/0277-5379(89)90348-9. [DOI] [PubMed] [Google Scholar]
- Murphy C. S., Pink J. J., Jordan V. C. Characterization of a receptor-negative, hormone-nonresponsive clone derived from a T47D human breast cancer cell line kept under estrogen-free conditions. Cancer Res. 1990 Nov 15;50(22):7285–7292. [PubMed] [Google Scholar]
- Paik S., Hartmann D. P., Dickson R. B., Lippman M. E. Antiestrogen resistance in ER positive breast cancer cells. Breast Cancer Res Treat. 1994;31(2-3):301–307. doi: 10.1007/BF00666162. [DOI] [PubMed] [Google Scholar]
- Pink J. J., Bilimoria M. M., Assikis J., Jordan V. C. Irreversible loss of the oestrogen receptor in T47D breast cancer cells following prolonged oestrogen deprivation. Br J Cancer. 1996 Oct;74(8):1227–1236. doi: 10.1038/bjc.1996.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink J. J., Jiang S. Y., Fritsch M., Jordan V. C. An estrogen-independent MCF-7 breast cancer cell line which contains a novel 80-kilodalton estrogen receptor-related protein. Cancer Res. 1995 Jun 15;55(12):2583–2590. [PubMed] [Google Scholar]
- Pink J. J., Jordan V. C. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res. 1996 May 15;56(10):2321–2330. [PubMed] [Google Scholar]
- Pink J. J., Wu S. Q., Wolf D. M., Bilimoria M. M., Jordan V. C. A novel 80 kDa human estrogen receptor containing a duplication of exons 6 and 7. Nucleic Acids Res. 1996 Mar 1;24(5):962–969. doi: 10.1093/nar/24.5.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponglikitmongkol M., Green S., Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J. 1988 Nov;7(11):3385–3388. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyser M. Mutations in the estrogen receptor gene. Hum Mutat. 1995;6(2):97–103. doi: 10.1002/humu.1380060202. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. L., Mangel W. F., Katzenellenbogen B. S. Biological activities of tamoxifen aziridine, an antiestrogen-based affinity label for the estrogen receptor, in vivo and in vitro. J Steroid Biochem. 1985 Dec;23(6A):875–881. doi: 10.1016/0022-4731(85)90042-1. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Jordan V. C. Adaptation of estrogen-dependent MCF-7 cells to low estrogen (phenol red-free) culture. Eur J Cancer Clin Oncol. 1987 Dec;23(12):1935–1939. doi: 10.1016/0277-5379(87)90062-9. [DOI] [PubMed] [Google Scholar]