Figure 1.
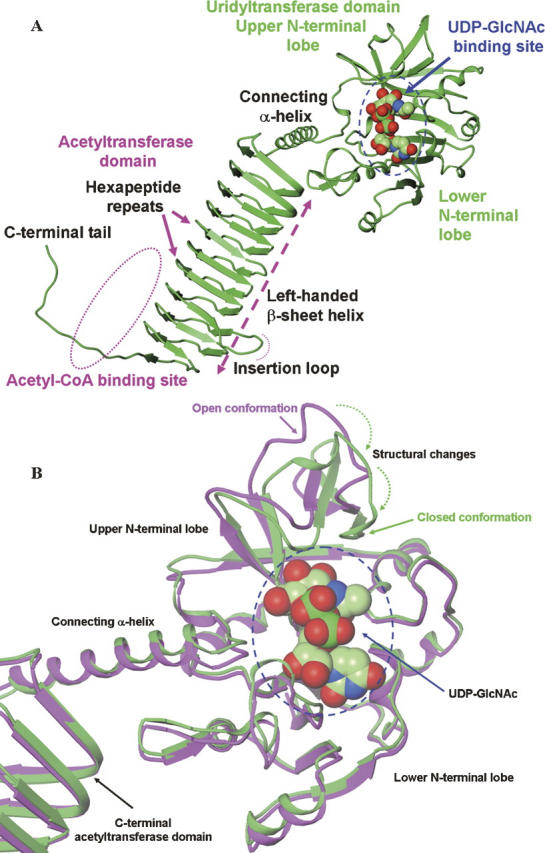
Structures of hiGlmU. (A) Ribbon representation of the bifunctional hiGlmU in a complex with the UDP-GlcNAc product bound at the uridyltransferase-binding site of the N-terminal urydiltransferase domain. (B) Structural changes at the uridyltransferase active site of hiGlmU upon the UDP-GlcNAc-binding event. Ribbon representation of the protein coordinates of hiGlmU in a complex with UDP is colored in magenta. In the UDP-bound complex, the uridyltrasnferase active site is in open conformation similar to the structure of hiGlmU in apo form. Ribbon representation of hiGlmU in a complex with UDP-GlcNAc is colored in green. Upon the substrate binding, the upper lobe of the uridyltransferase domain forms a closed conformation by tilting toward the lower N-terminal lobe and anchoring GlcNAc of UDP-GlcNAc (shown by two dotted arrows). The UDP-GlcNAc molecule is colored in the following atom colors: carbon, light green; nitrogen, blue; oxygen, red; phosphorus, dark green. UDP molecule is omitted for clarity.
