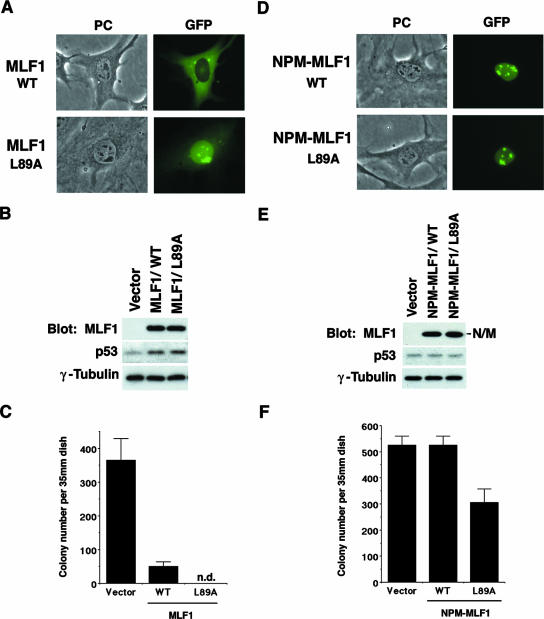
FIG. 3.
Disruption of NES enhances MLF1-induced growth arrest. NIH 3T3 cells (Arf null, p53 WT) were transfected with GFP alone, the GFP-fused WT (MLF1 or NPM-MLF1), and the NES mutant (MLF1/L89A or NPM-MLF1/L89A [leucine 89 of MLF1 sequence is replaced by alanine]) expression vectors and selected in puromycin for 5 days. (A) Subcellular localization of MLF1/L89A. Before puromycin selection, GFP-positive cells were viewed using fluorescence microscopy and photographed. PC, phase contrast. (B) Expression levels of GFP-MLF1, GFP-MLF1/L89A, and endogenous p53 proteins at an early phase are shown by Western blotting with antibodies to MLF1, p53, and γ-tubulin. (C) At 14 days posttransfection, GFP-positive colonies were enumerated. Data are the averages of three independent experiments shown as means ± standard deviations (SD). (D) Subcellular localization of NPM-MLF1 and NPM-MLF1/L89A. Before puromycin selection, GFP-positive cells were viewed using fluorescence microscopy and photographed. (E) Expression levels of the GFP-NPM-MLF1, GFP-NPM-MLF1/L89A, and p53 proteins at an early phase are shown by Western blotting with antibodies to MLF1, p53, and γ-tubulin. (F) At 14 days posttransfection, GFP-positive colonies were enumerated. Data are averages of three independent experiments shown as means ± SD. Results shown in panels B and E are representative of three independent experiments.