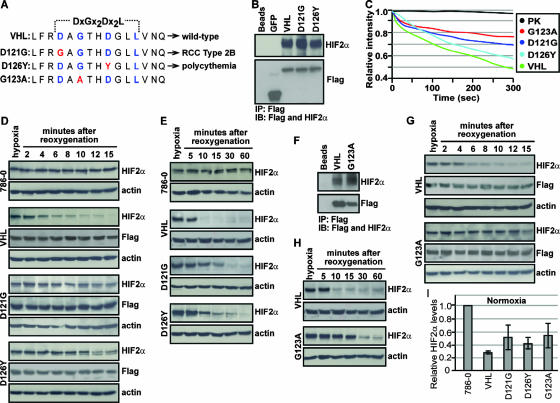
FIG. 7.
Cancer-causing mutations within TD-NEM of VHL abrogate nuclear export and oxygen-dependent degradation of HIF2α. (A) Sequence alignment depicting cancer-causing mutations (red) in the key TD-NEM residues of VHL that lead to RCC type 2B (D121G) and polycythemia (D126Y) in humans. G123A, which exhibits a defect in nuclear export as shown in Fig. 6, was also used to test its ability to mediate HIF degradation. (B) D121G and D126Y retain the ability to bind to HIF. Cells stably expressing Flag-tagged VHL-GFP, D121G-GFP, D126Y-GFP, and GFP were placed under hypoxic conditions and treated with 10 μM MG132 for 2 h before being harvested. Cell lysates were immunoprecipitated with anti-Flag beads and immunoblotted with anti-Flag and anti-HIF2α antibodies. (C) Cancer-causing mutants D121G and D126Y in TD-NEM decrease the nuclear export activity of VHL. Cells stably expressing VHL-GFP, D121G-GFP, D126Y-GFP, or G123A-GFP or transiently expressing PK-GFP-NLS were submitted to cytoplasmic FLIP as described previously. The loss of nuclear fluorescence was monitored and plotted on a graph. (D and E) Cells expressing D121G or D126Y exhibit a deficiency in HIF degradation. Stable cell lines of VHL-GFP, D121G-GFP, D126Y-GFP, and the VHL-defective cell line 786-0 were incubated for 20 h under hypoxic conditions before being reoxygenated by placing them in a normoxic environment, for the indicated time. Cells were lysed with 4% SDS and submitted to Western blot analysis using an anti-HIF2α antibody. Levels of VHL or its mutant counterparts were monitored using anti-Flag antibody, and actin was used to ensure equal loading of lysates. (F) G123A retains the ability to bind to HIF. Cells stably expressing the VHL point mutant, G123A, were treated the same as described for panel B. (G and H) Cells expressing G123A exhibit a deficiency in HIF degradation. G123A stably expressing cells were treated in the same manner as described for panels D and E. (I) D121G, D126Y, and G123A stable cells express higher normoxic HIF levels compared to wild-type VHL. Stable cell lines incubated under normoxic conditions were lysed with 4% SDS and submitted to Western blot analysis using anti-HIF2α and antiactin antibodies. HIF2α levels were normalized to actin and values, calculated relative to HIF2α levels in 786-0 cells, were plotted on a graph.