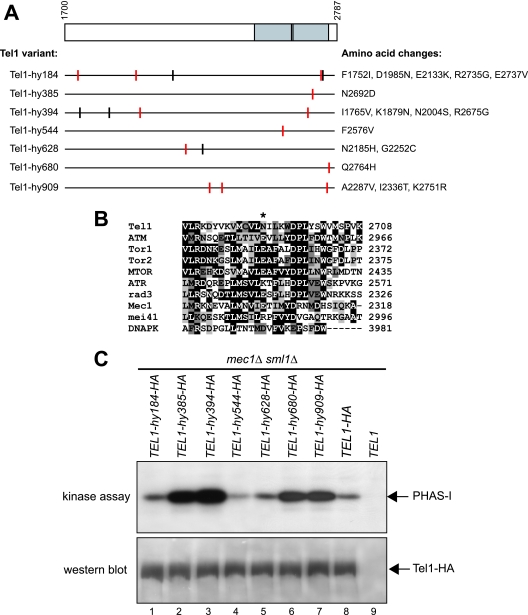
FIG. 2.
Tel1-hy amino acid changes and in vitro kinase activity. (A) The C-terminal part of wild-type Tel1, spanning amino acid residues 1700 to 2787, is schematically depicted in the top part, with a gray box highlighting the C-terminal domain (between amino acids 2460 and 2755) that is shared by a number of PI3-like kinases, including mammalian ATM, ATR, DNA-PK, and MTOR; S. pombe Tel1 and Rad3; and S. cerevisiae Tel1, Mec1, and Tor1. Black lines inside the gray block indicate the G2611, D2612, N2616, and D2631 amino acid residues that have been shown to be essential for Tel1 kinase activity (25). Black horizontal lines represent the same C-terminal region in the Tel1-hy variants indicated on the left, with the corresponding amino acid changes listed on the right. Vertical lines indicate the position of the amino acid changes, with red lines highlighting changes affecting conserved amino acid residues based on a ClustalW alignment of the whole Tel1 and human ATM amino acid sequences. (B) ClustalW alignment of Tel1 amino acid sequence between residues 2679 and 2708, spanning the conserved PI3 kinase domain, with the corresponding sequences of PI3-like kinases indicated on the left. Identical amino acids are indicated by black boxes, and similar ones are indicated by different shades of gray. An asterisk indicates the position corresponding to the N2692D alteration in Tel1-hy385. (C) Immunoprecipitation and in vitro kinase assays of HA-tagged Tel1 and Tel1-hy proteins. The strains used were TEL1-hy184-HA (YLL2104), TEL1-hy385-HA (YLL1974), TEL1-hy394-HA (YLL1975), TEL1-hy544-HA (YLL1976), TEL1-hy628-HA (YLL1977), TEL1-hy680-HA (YLL1978), TEL1-hy909-HA (YLL1979), TEL1-HA (DMP4690/9A), and TEL1 (YLL490), all carrying the deletions of both MEC1 and SML1. Kinase assays and Western blot analysis (see Materials and Methods) were performed on equal amounts of anti-HA immunoprecipitates of protein extracts from exponentially growing untreated cells. Products of a kinase reaction using γ-32P-labeled ATP were analyzed by SDS-polyacrylamide gel electrophoresis (kinase assay). All of the immunoprecipitates also were subjected to Western blot analysis using anti-HA antibodies (western blot).