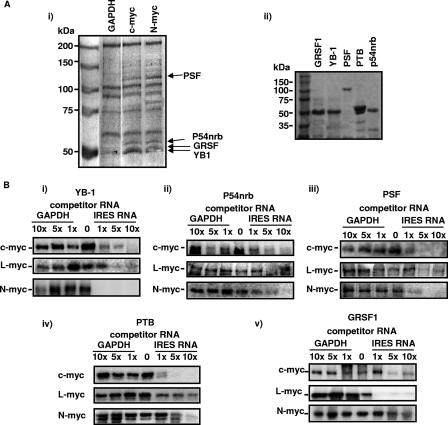
FIG. 1.
PSF, p54nrb, GRSF-1, and YB-1 bind specifically to the myc IRESs. (Ai) Biotinylated RNAs were incubated with HeLa cell extracts, and protein-RNA complexes were isolated on streptavidin magnetic beads. Proteins were separated by PAGE and identified by LC-MS-MS. (Aii) Proteins were subcloned and overexpressed in E. coli. A polyacrylamide gel of the purified proteins used in subsequent experiments is shown. (B) UV cross-linking assays were performed using radiolabeled c-, L- or N-myc IRES RNAs, which were incubated with 0.2 μg of YB-1 (i), p54nrb (ii), PSF (iii), PTB (iv), or GRSF-1 (v) in the presence/absence of unlabeled GAPDH RNA or IRES RNA. Products were separated on a 10% polyacrylamide gel. In all cases, unlabeled IRES RNA competed for binding, while no competition was observed with GAPDH RNA, even at a 10-fold (10×) molar excess.