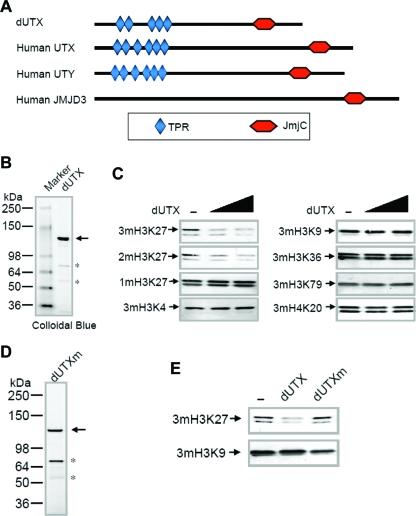
FIG. 1.
The Drosophila homolog of UTX is an H3 lysine 27 demethylase. (A) Schematic representation of the domain organization of Drosophila UTX (CG5640) and its human homologs. Members of the UTX family have a conserved JmjC domain at the C terminus and 5 to 6 tetratricopeptide repeats (TPR) at the N terminus. Human UTX and UTY are X and Y chromosome-linked homologs, respectively, from a pseudoautosomal portion of the sex chromosomes and are therefore highly similar. Since the Y-linked copy of such X-Y pairs generally mutates at a higher rate (13), the X-linked copy, UTX, can be considered more orthologous to the Drosophila homolog. dUTX shares higher overall sequence identity with UTX than with UTY or JMJD3. (B) Expression of recombinant dUTX in a baculovirus system. N-terminally Flag-tagged dUTX was purified from SF21 cells. Asterisks indicate nonspecific polypeptides. (C) Different amounts of recombinant dUTX (1 and 2 μg) were used in histone demethylation assays using bulk histones as substrates, and reaction mixtures were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by Western blotting with antibodies to various sites of histone methylation. 1m, monomethylated; 2m, dimethylated; 3m, trimethylated. (D) Expression of a mutant version of dUTX (dUTXm) that bears double mutations (H883A and E885A) in the predicted iron-binding sites of the JmjC domain. Recombinant dUTXm was purified as described for panel B. (E) Comparison of H3K27 demethylase activities with 2 μg each of dUTX or dUTXm. Only the wild-type version of dUTX can demethylate H3K27me3, indicating that the observed enzymatic activity is intrinsic to the dUTX polypeptide.