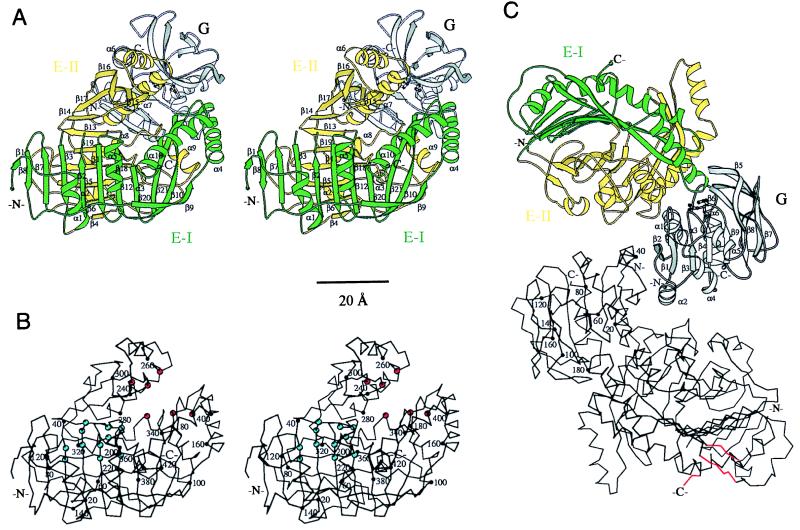
Figure 3.
Three-dimensional structure of anthranilate synthase of S. solfataricus. Residues 33–39 (loop β2β3) of the TrpE subunit are missing in the model. (A) Ribbon representation of the TrpG:TrpE protomer of anthranilate synthase. Domains I and II of the TrpE subunit are colored green and yellow, respectively. The secondary structural elements of TrpE are numbered. In the TrpG subunit (gray), the catalytic residues Cys-84, His-175, and Glu-177 are depicted in ball-and-stick. (B) Cα trace of the TrpE subunit. Every 20th residue (●) is numbered. Residues involved in catalysis and feedback inhibition by tryptophan are labeled by red and blue spheres, respectively. (C) The TrpG2:TrpE2 heterotetramer viewed along the crystallographic (and molecular) twofold axis. The top TrpG:TrpE protomer is depicted as ribbon diagram. The secondary structural elements of the TrpG subunit (gray) are numbered, and the catalytic residues Cys-84, His-175, and Glu-177 are depicted in ball-and-stick. The bottom protomer is given as a Cα trace, in which every 20th residue (●) of the TrpG subunit is numbered. Regions of the TrpE subunit involved in a crystal lattice contact through an additional crystallographic twofold axis are marked in red. Drawings produced with molscript (23).