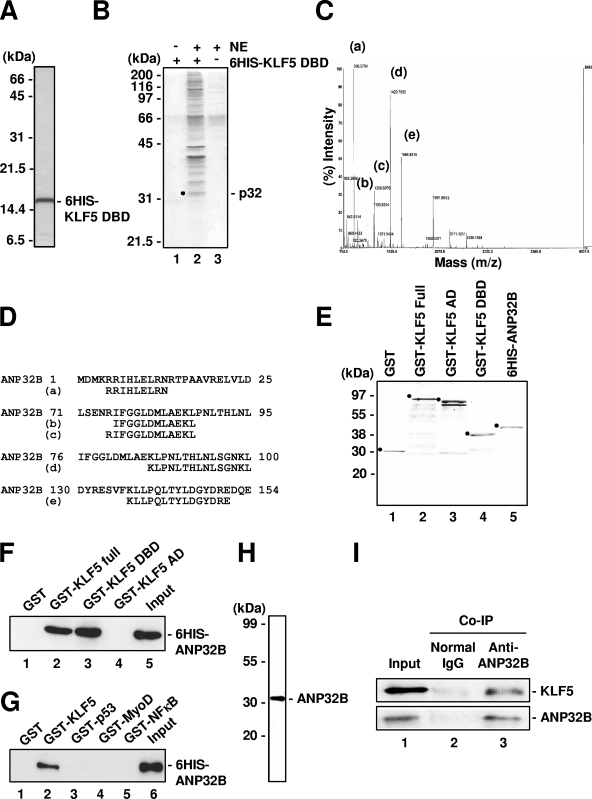
FIG. 1.
Isolation of ANP32B as an interactor with KLF5. (A) Silver-stained gel of 6HIS-KLF5-ZF/DBD. (B) Isolation of factors associating with KLF5-ZF/DBD. Lane 3 is HeLa S3 nuclear extract (NE). p32, 32-kDa band. (C) MALDI-TOF mass spectra obtained from tryptic peptides of p32. Fragment peaks assigned to ANP32B are labeled. (D) Partial peptide sequences of human ANP32B. Numbering is from the initiation methionine of ANP32B. The peptide sequences of peaks a to e obtained by peptide mass fingerprinting are shown. (E) Coomassie blue-stained gel of the recombinant proteins used for protein-protein interaction studies. (F) In vitro binding of KLF5 full-length protein and ANP32B. Bound proteins were detected by anti-His antibody. (G) In vitro binding of KLF5 ZF/DBD and ANP32B. Bound proteins were detected by anti-His antibody. (H) Specific detection of target protein by anti-ANP32B antibody. HeLa whole-cell extract was analyzed by Western blotting using prepared rabbit polyclonal anti-ANP32B. (I) Cellular binding of ANP32B and KLF5. Bound proteins were detected by anti-KLF5 (KM1785) and anti-ANP32B (rabbit polyclonal) antibodies.