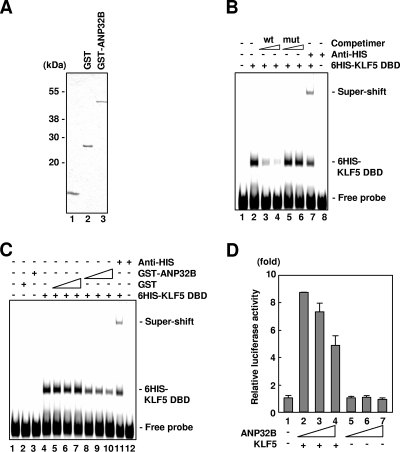
FIG. 2.
Negative regulation of KLF5 activities by ANP32B. (A) Coomassie blue-stained gel of the recombinant proteins used for DNA-protein binding studies. (B) Sequence-specific DNA binding of KLF5-ZF/DBD. wt and mut represent wild-type and mutant oligonucleotide competimers, respectively (lanes 3 and 4 and lanes 5 and 6, respectively) (1 and 4 pmol from left to right). Two picomoles of 6HIS-KLF5-ZF/DBD was used. The 6HIS-KLF5-ZF/DBD protein was supershifted by 1 μg of anti-His antibody (lanes 7 and 8). (C) Effect of ANP32B on DNA-binding activity of KLF5-ZF/DBD. Thirty nanograms of 6HIS-KLF5-ZF/DBD was used. The 6HIS-KLF5-ZF/DBD protein was supershifted by 1 μg of anti-His probe antibody (lanes 7 and 8). The amounts of recombinant proteins were as follows; 1.2, 2.4, and 4.8 μg of GST (lanes 5, 6, and 7, respectively) and GST-ANP32B (lanes 8, 9, and 10, respectively); 4.8 μg of GST (lane 2) and GST-ANP32B (lane 3); and the presence (lanes 4 to 11) or absence (lanes 1 to 3 and 12) of 30 ng of 6HIS-KLF5-ZF/DBD. (D) Effect of ANP32B on KLF5 transactivation. Effectors were as follows; lanes 2, 3, and 4 were 0.5 μg of pCAG-KLF5, respectively, and lanes 3 and 4 and lanes 6 and 7 were 0.25 μg and 0.5 μg of pcDNA4-ANP32B, respectively. Error bars denote standard errors.