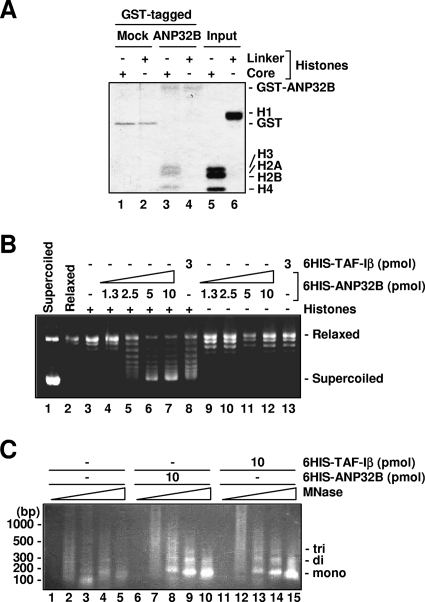
FIG. 3.
Characterization of the novel histone chaperone ANP32B. (A) In vitro binding of ANP32B and core histones. Bound proteins were detected by Coomassie blue staining. (B) In vitro nucleosome assembly activity of ANP32B. Thirty-five nanograms of 6HIS-ANP32B and 45 ng of 6HIS-TAF-Iβ are equivalent to 1 pmol. Reaction mixtures were incubated in the presence (+) or in the absence (−) of histones with recombinant proteins, whose amounts are indicated. 6HIS-TAF-Iβ was used as a positive control for in vitro nucleosome assembly activity. (C) ANP32B-dependent nucleosome formation in vitro. Reactions were done with histones in either the presence or the absence of indicated factors. Each sample was treated with MNase (0, 6.4, 32, 160, and 800 units/ml from left to right). 6HIS-TAF-Iβ was used as a positive control for in vitro nucleosome assembly activity.