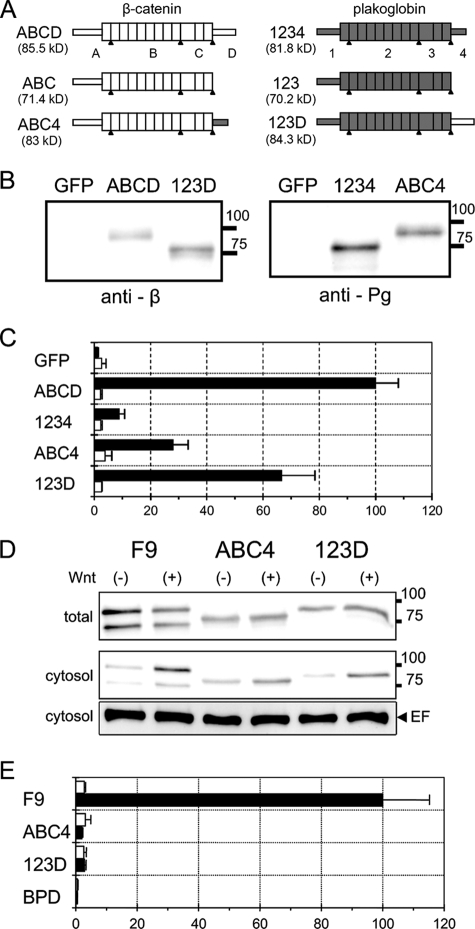
FIG. 7.
LEF/TCF-dependent transcriptional activity in BPD cells transiently expressing β-catenin/plakoglobin chimeric molecules. (A) Schematic representation of the deletion and chimeric constructs of β-catenin and plakoglobin. The proteins are conceptually divided into four regions (see Materials and Methods for details). ABCD and 1234 are intact β-catenin and plakoglobin, respectively. ABC and 123 are C-terminal deleted β-catenin and plakoglobin, respectively. In ABC4 and 123D, the C-terminal domain of β-catenin and plakoglobin were interchanged. Portions of β-catenin and plakoglobin are represented as open and shaded boxes, respectively. The expected molecular mass of each protein is shown in parentheses. The apparent molecular masses of molecules including ABC and 123 are a little higher and lower, respectively, than the expected ones. (B) Expression of β-catenin/plakoglobin chimeras in BPD cells. Cells were transfected with expression vectors for β-catenin (ABCD), plakoglobin (1234), and two chimeric molecules, ABC4 and 123D. As a control, the expression vector for GFP was used. Western blots of total cell lysates were probed with anti-β-catenin (anti-β) or antiplakoglobin (anti-Pg) antibodies. The positions of molecular weight markers (in thousands) are indicated on the right. (C) Effect of β-catenin/plakoglobin chimeric molecules on TOPflash (closed bars) or FOPflash (open bars) luciferase reporter activity. Cells were transiently cotransfected with pTOPflash (or FOPflash) and pCMV-renilla luciferase reporters and, where indicated, with expression vectors for β-catenin (ABCD), plakoglobin (1234), and the chimeric molecules, ABC4 and 123D. The value for BPD cells transfected with β-catenin (ABCD) was set to 100. Error bars indicate standard deviations. (D) Expression of β-catenin and plakoglobin in wild-type F9 cells and of 123D and ABC4 in BPD transfectants. Cells were treated with conditioned medium from L cells expressing Wnt-3a (+) or with that from parental L cells (−) for 15 h. In the upper panel (total) and the middle panel (cytosol), Western blots of total cell lysate and cytosolic fraction, respectively, were probed with a mixture of anti-β-catenin and antiplakoglobin antibodies. The positions of molecular weight markers (in thousands) are indicated on the right. Eukaryotic elongation factor 2 (EF) levels were used as an internal control (lower panel). (E) Effect of Wnt-3a on TOPflash luciferase reporter activity in F9, BPD, and BPD cells stably expressing ABC4 and 123D. Cells were transiently transfected with pTOPflash and pCMV-renilla luciferase reporters and then treated with conditioned medium from L cells expressing Wnt-3a (black bars) or with that from parental L cells (white bars) for 15 h. The value for F9 cells treated with Wnt-3a was set at 100.