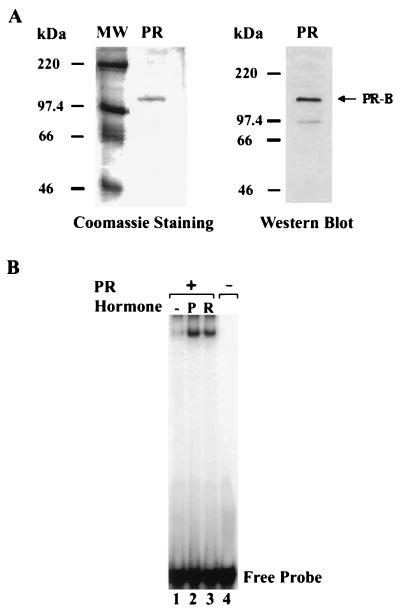
Figure 1.
Purification of human PRB from baculovirus-infected Sf9 cells. (A) The full-length His6-tagged PRB was overexpressed in Sf9 cells by using a baculovirus expression system and purified by Ni-NTA affinity chromatography. The recombinant protein was analyzed on a 10% polyacrylamide/SDS gel and then subjected to staining with Coomassie brilliant blue R-250 (Left) or Western blot analysis with a polyclonal antibody against PR (Right). (B) DNA-binding activity of purified PRB. Gel retardation experiments were carried out as previously described (31). Recombinant PRB was preincubated with the control buffer (lane 1), 10−7 M progesterone (P, lane 2), or R5020 (R, lane 3) for 15 min at 25°C. Labeled double-stranded PRE oligonucleotides were then added and the reaction mixture was incubated for an additional 15 min. Samples were analyzed on a nondenaturing 5% polyacrylamide gel.