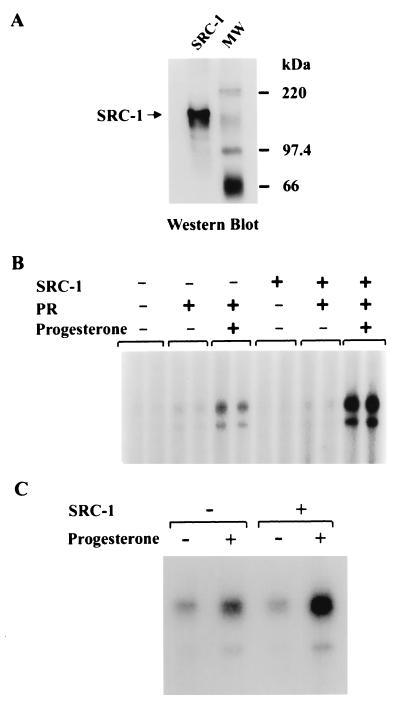
Figure 4.
SRC-1 functions as a coactivator of PR in vitro. (A) Expression and purification of SRC-1. Flag-tagged SRC-1 was expressed in injected Xenopus oocytes and affinity-purified with a monoclonal antibody against the Flag epitope. The purified recombinant protein was analyzed on a 7.5% polyacrylamide/SDS gel and subjected to Western blot analysis using a polyclonal antibody against SRC-1. (B) SRC-1 enhances ligand-dependent transcription by purified PRB on chromatin in vitro. pPRE3-E4 was assembled into chromatin. Where indicated, purified PRB, progesterone, and SRC-1 were added to preassembled chromatin, followed by a 30-min incubation at 27°C. The samples were then subjected to in vitro transcription analysis. The final concentrations of PRB, progesterone, and SRC-1 in the transcription reactions were 15 nM, 10−7 M, and 1 nM, respectively. All reactions were performed in duplicate. (C) SRC-1 stimulates PR-dependent transcription from nonchromatin template in vitro. In vitro transcription was performed with pPRE3-E4 DNA template in hormone-untreated or hormone-treated T47D nuclear extract (10 μg) in the presence or absence of exogenous SRC-1, as noted. The final concentration of SRC-1 in the transcription reactions was 1 nM.