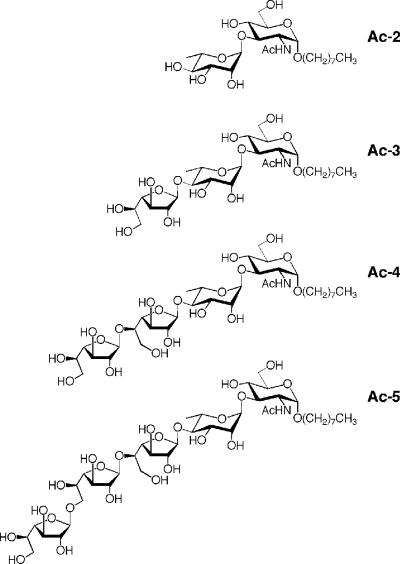
FIG. 2.
Structures of the synthetic acceptors Ac-2, Ac-3, Ac-4, and Ac-5. Oligosaccharide portions of Ac-2 to Ac-5 match the structures of the natural intermediates in galactan biosynthesis, namely GL-2, GL-3, GL-4, and GL-5. Abbreviations: Ac-2, octyl α-l-rhamnopyranosyl-(1→3)-2-acetamido-2-deoxy-α-d-glucopyranoside; Ac-3, octyl β-d-galactofuranosyl-(1→4)-α-l-rhamnopyranosyl-(1→3)-2-acetamido-2-deoxy-α-d-glucopyrano-side; Ac-4, octyl β-d-galactofuranosyl-(1→5)-β-d-galactofuranosyl-(1→4)-α-l-rhamnopyranosyl-(1→3)-2-acetamido-2-deoxy-α-d-glucopyranoside; Ac-5, octyl β-d-galactofuranosyl-(1→6)-β-d-galactofuranosyl-(1→5)-β-d-galactofuranosyl-(1→4)-α-l-rhamnopyranosyl-(1→3)-2-acetamido-2-deoxy-α-d-glucopyranoside. Synthesis of these acceptors will be described by Lowary et al. (unpublished data).