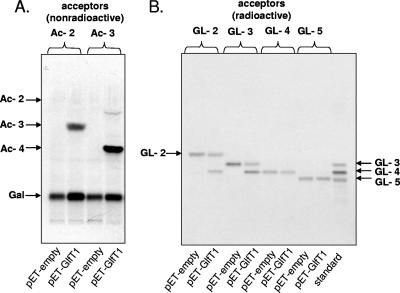
FIG. 3.
Recognition of the dual β-(1→4) and β-(1→5) Galf transferase activities of GlfT1. The synthetic acceptors Ac-2 and Ac-3 (A) and the native substrates GL-2, GL-3, GL-4, and GL-5 (B) were used as the acceptors of galactofuranosyl residues in the reaction catalyzed by GlfT1 expressed in the heterologous host, E. coli. The positions of Ac-2, Ac-3, and Ac-4 substrates on TLC plate were visualized by staining with α-naphthol.