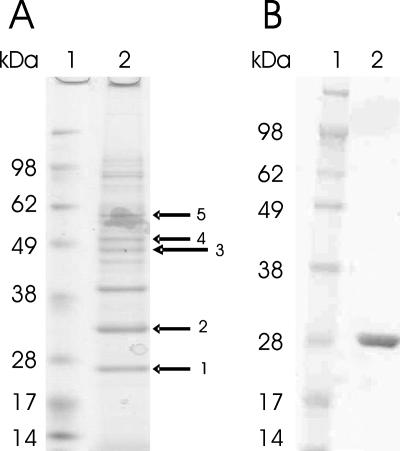
FIG. 1.
(A) SDS-PAGE of C. glutamicum proteins eluted in a DNA affinity chromatography experiment with TGED buffer containing 2 M NaCl using the NCgl2816-lldD promoter as a probe and cell extracts from l-lactate-grown cells of C. glutamicum WT (lane 2). MALDI-TOF mass spectrometry of tryptic peptides from the protein bands revealed LldR (arrow 1), RamA (NCgl2472) (arrow 2), a subunit of DNA polymerase I (NCgl1299) (arrow 3), a subunit of DNA polymerase III (NCgl2035) (arrow 4), and a subunit of a putative restriction nuclease (NCgl1705) (arrow 5). The prominent band between arrows 2 and 3 could not be identified. Lane 1 contained protein standards. (B) SDS-PAGE of purified LldR. Lane 2 contained purified LldR with a His tag, and lane 1 contained protein standards.