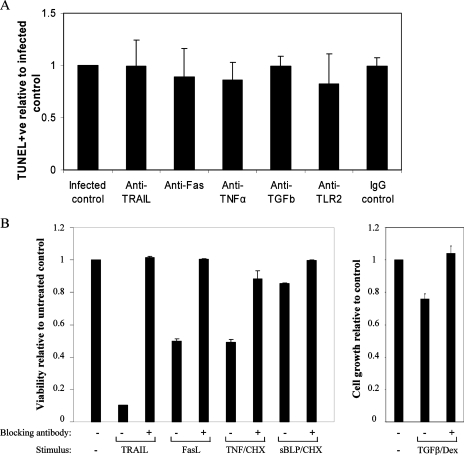
FIG. 4.
Mechanism of bystander macrophage apoptosis is independent of TRAIL, Fas, TNF-α, TGFβ, and TLR2. (A) Macrophages were treated with anti-Fas antibody (neutralizing; ZB4; 2.5 μg/ml), anti-TNF-α antibody (10 ng/ml), anti-TGFβ antibody (2.5 μg/ml), anti-TLR2 antibody (2.5 μg/ml), or an IgG1 isotype control (10 ng/ml) before and after infection with M. tuberculosis strain H37Ra (MOI, 1 to 5). At 72 h postinfection a TUNEL analysis was performed, and apoptosis levels were assessed using fluorescent microscopy. There was no significant difference between control bystander apoptosis levels and the apoptosis levels of preparations treated with neutralizing antibodies. (B) As positive controls for the neutralizing antibody experiments, Jurkat cells were incubated in the presence or absence of Fas ligand (10 ng/nl), TRAIL (20 ng/ml), and TNF-α (5 ng/ml) and/or cycloheximide (CHX) (0.2 μg/ml) for 48 h with or without the appropriate neutralizing antibody at the concentrations described above for panel A; the TLR2 antibody was tested to determine its ability to block apoptosis of THP-1 cells induced by the synthetic bacterial lipopeptide (sBLP) Pam3CysSerLys4 (20 ng/ml) in combination with cycloheximide (0.5 μg/ml). The cells were then stained with propidium iodide (50 μg/ml) and analyzed by flow cytometry to measure apoptosis. To validate the neutralizing effect of the anti-TFGβ antibody, THP-1 cells were incubated with dexamethasone (Dex) (25 nM) or dexamethasone plus TGFβ (2 ng/ml) for 72 h in the presence or absence of anti-TGFβ. Cell counts were determined by microscopy using a hemocytometer. The values are the means and standard errors of the means for three experiments, and the results are expressed relative to the appropriate control for each stimulus.