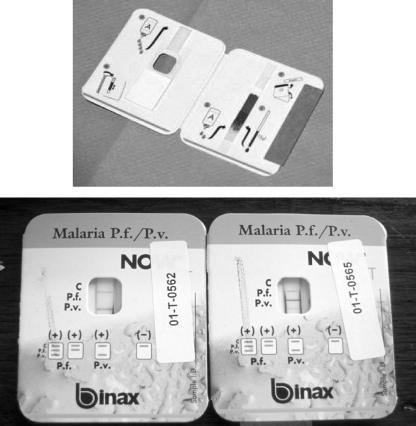
FIG. 2.
Example of the only U.S. FDA-approved MRDT product, BinaxNOW Malaria (Binax, Inc., Inverness Medical Professional Diagnostics, Scarborough, ME (note that the package design has changed from the pictured version)). This assay is based on detection of the antigens HRP-2 (for P. falciparum) and aldolase (for generic Plasmodium). Per the package insert, the overall sensitivity and specificity are 95.3% and 94.2%, respectively, for P. falciparum, and 68.9% and 99.8%, respectively, for P. vivax. (Top) Card undergoing sample wicking up the nitrocellulose strip after application of the blood and reagent. (Bottom) Example of two cards. The left card has a positive control line and a positive generic Plasmodium line, while the right card has a positive control line, a positive P. falciparum line, and a positive generic Plasmodium line.