Abstract
Against 447 anaerobe strains, the investigational carbapenem doripenem had an MIC50 of 0.125 μg/ml and an MIC90 of 1 μg/ml. Results were similar to those for imipenem, meropenem, and ertapenem. Time-kill studies showed that doripenem had very good bactericidal activity compared to other carbapenems, with 99.9% killing of 11 strains at 2× MIC after 48 h.
Anaerobes are well-recognized human pathogens, and drug resistance in this organism group is common. Among the Bacteroides fragilis group, all species except Bacteroides distasonis regularly produce β-lactamases. β-Lactamase production is also common among Prevotella and Porphyromonas spp. and is also found in some fusobacteria. Anaerobic gram-positive cocci and some gram-negative rods may be resistant to clindamycin, and metronidazole is inactive against many of the gram-positive non-spore-forming rods (1-4, 10).
Doripenem (formerly S-4661) is an investigational parenteral 1-β-carbapenem with a molecular structure that confers β-lactamase stability and resistance to inactivation by renal dehydropeptidases. The characteristics of doripenem include a spectrum and potency against gram-positive and gram-negative cocci, which are most similar to those of imipenem, and activity against gram-negative organisms, which is most similar to that of meropenem. A particular feature, attributed to the side chain at position 2, is greater activity against multiresistant gram-negative nonfermenters (except for Stenotrophomonas maltophilia) than is usually found (5, 7, 8, 11, 12, 17).
This study (i) used standardized MIC techniques to test the susceptibilities of 447 gram-positive and -negative anaerobes, most isolated from patients with intra-abdominal infections and pelvic inflammatory disease, to doripenem, compared to their susceptibilities to imipenem, meropenem, ertapenem, piperacillin-tazobactam, amoxicillin-clavulanate, ceftriaxone, clindamycin, and metronidazole, and (ii) tested the activities of the above-named drugs against 14 selected gram-positive and -negative anaerobes by time-kill methodology. Drugs other than the three carbapenems were chosen to represent current standard therapies for anaerobic infections for comparison purposes.
Most (>70%) of the commonly encountered anaerobic organisms tested (Table 1) were isolated within the past 4 years, and most were isolated from patients with intra-abdominal and pelvic inflammatory infections. All strains were identified to the species level using conventional methodology and the latest taxonomic criteria (13) and were stored at −70°C in double-strength skim milk (Becton, Dickinson and Company, Sparks, MD) prior to testing. Doripenem powder was obtained from Johnson and Johnson Pharmaceutical Research and Development, Raritan, NJ, and other drugs were obtained from their respective manufacturers.
TABLE 1.
MICs of agents against anaerobes
| Organisma | Drug(s) | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Bacteroides fragilis (69/70) | Doripenem | 0.125-8 | 0.25 | 1 |
| Imipenem | 0.03-2 | 0.125 | 0.25 | |
| Meropenem | 0.06-8 | 0.125 | 1 | |
| Ertapenem | 0.125-8 | 0.25 | 2 | |
| Piperacillin-tazobactam | ≤0.125-8 | 0.5 | 1 | |
| Amoxicillin-clavulanate | 0.25-4 | 0.5 | 2 | |
| Ceftriaxone | ≤0.125->128 | 64 | >128 | |
| Clindamycin | 0.125->16 | 1 | 4 | |
| Metronidazole | 0.25-2 | 1 | 1 | |
| Bacteroides thetaiotaomicron (30/30) | Doripenem | 0.25-0.5 | 0.25 | 0.5 |
| Imipenem | 0.25-0.5 | 0.25 | 0.5 | |
| Meropenem | 0.125-0.5 | 0.25 | 0.5 | |
| Ertapenem | 0.5-2 | 0.5 | 1 | |
| Piperacillin-tazobactam | 2-32 | 16 | 16 | |
| Amoxicillin-clavulanate | 0.5-8 | 1 | 1 | |
| Ceftriaxone | 64->128 | >128 | >128 | |
| Clindamycin | 0.25->16 | 4 | 8 | |
| Metronidazole | 0.25-2 | 1 | 1 | |
| Bacteroides ovatus (10/10) | Doripenem | 0.25-4 | 0.25 | 1 |
| Imipenem | 0.125-2 | 0.25 | 0.5 | |
| Meropenem | 0.25-4 | 0.25 | 0.5 | |
| Ertapenem | 0.5-8 | 0.5 | 2 | |
| Piperacillin-tazobactam | 4-16 | 4 | 8 | |
| Amoxicillin-clavulanate | 0.5-8 | 0.5 | 2 | |
| Ceftriaxone | 64->128 | 64 | >128 | |
| Clindamycin | 2->16 | 2 | >16 | |
| Metronidazole | 0.5-2 | 1 | 2 | |
| Bacteroides vulgatus (20/20) | Doripenem | 0.125-1 | 0.25 | 1 |
| Imipenem | 0.06-1 | 0.5 | 1 | |
| Meropenem | 0.125-1 | 0.25 | 1 | |
| Ertapenem | 0.06-2 | 0.125 | 2 | |
| Piperacillin-tazobactam | 0.5-64 | 8 | 16 | |
| Amoxicillin-clavulanate | 0.5-8 | 0.5 | 8 | |
| Ceftriaxone | 2->128 | 16 | >128 | |
| Clindamycin | ≤0.016->16 | 0.03 | 0.5 | |
| Metronidazole | ≤0.125-1 | 0.5 | 1 | |
| Bacteroides distasonis (8/20) | Doripenem | 0.25-1 | 0.5 | 1 |
| Imipenem | 0.25-1 | 0.5 | 1 | |
| Meropenem | 0.125-1 | 0.125 | 0.5 | |
| Ertapenem | 0.5-2 | 0.5 | 1 | |
| Piperacillin-tazobactam | 4-8 | 4 | 8 | |
| Amoxicillin-clavulanate | 1-8 | 2 | 8 | |
| Ceftriaxone | 1->128 | 8 | >128 | |
| Clindamycin | 0.03->16 | 4 | >16 | |
| Metronidazole | 1-2 | 1 | 1 | |
| Prevotella bivia (21/30) | Doripenem | 0.06-0.25 | 0.06 | 0.125 |
| Imipenem | ≤0.016-0.125 | 0.03 | 0.06 | |
| Meropenem | 0.03-0.25 | 0.06 | 0.125 | |
| Ertapenem | 0.06-0.5 | 0.25 | 0.25 | |
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-4 | 0.25 | 4 | |
| Ceftriaxone | ≤0.125-128 | 8 | 64 | |
| Clindamycin | ≤0.016->16 | 0.03 | >16 | |
| Metronidazole | 0.5-4 | 2 | 2 | |
| Prevotella disiens (7/10) | Doripenem | 0.03-0.125 | 0.06 | 0.125 |
| Imipenem | 0.03-0.125 | 0.03 | 0.125 | |
| Meropenem | 0.03-0.125 | 0.03 | 0.125 | |
| Ertapenem | 0.03-0.25 | 0.06 | 0.25 | |
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-2 | ≤0.125 | 0.5 | |
| Ceftriaxone | ≤0.125-64 | 2 | 64 | |
| Clindamycin | ≤0.016->16 | ≤0.016 | 0.03 | |
| Metronidazole | 0.5-2 | 0.5 | 2 | |
| Prevotella intermedia/Prevotella nigrescens (19/30) | Doripenem | ≤0.016-0.125 | 0.03 | 0.06 |
| Imipenem | ≤0.016-0.06 | 0.03 | 0.03 | |
| Meropenem | ≤0.016-0.125 | 0.03 | 0.06 | |
| Ertapenem | ≤0.016-0.125 | 0.03 | 0.06 | |
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-1 | ≤0.125 | 0.5 | |
| Ceftriaxone | ≤0.125-64 | 4 | 16 | |
| Clindamycin | ≤0.016->16 | ≤0.016 | ≤0.016 | |
| Metronidazole | 0.25-1 | 0.5 | 1 | |
| Prevotella melaninogenica/Prevotella denticola (16/20) | Doripenem | ≤0.016-0.25 | 0.03 | 0.125 |
| Imipenem | ≤0.016-0.125 | 0.03 | 0.06 | |
| Meropenem | ≤0.016-0.25 | 0.06 | 0.25 | |
| Ertapenem | 0.03-0.5 | 0.125 | 0.5 | |
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-4 | 0.25 | 2 | |
| Ceftriaxone | ≤0.125-128 | 4 | 64 | |
| Clindamycin | ≤0.016->16 | 0.03 | 0.03 | |
| Metronidazole | ≤0.125-1 | 0.5 | 1 | |
| Prevotella corporis (6/10) | Doripenem | 0.03-0.06 | 0.03 | 0.06 |
| Imipenem | ≤0.016-0.06 | 0.03 | 0.06 | |
| Meropenem | ≤0.016-0.06 | 0.03 | 0.06 | |
| Ertapenem | ≤0.016-0.125 | 0.03 | 0.06 | |
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-0.5 | ≤0.125 | 0.25 | |
| Ceftriaxone | ≤0.125-32 | 4 | 32 | |
| Clindamycin | ≤0.016-1 | ≤0.016 | 0.03 | |
| Metronidazole | 0.25-1 | 0.5 | 1 | |
| Prevotella buccae (3/10) | Doripenem | 0.06-0.125 | 0.06 | 0.125 |
| Imipenem | 0.03-0.06 | 0.03 | 0.03 | |
| Meropenem | 0.06-0.06 | 0.06 | 0.06 | |
| Ertapenem | 0.06-0.125 | 0.125 | 0.125 | |
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-2 | ≤0.125 | 1 | |
| Ceftriaxone | ≤0.125-64 | 0.25 | 4 | |
| Clindamycin | ≤0.016-0.03 | ≤0.016 | 0.03 | |
| Metronidazole | 0.5-2 | 1 | 1 | |
| Porphyromonas asaccharolytica (0/4) | Doripenem | ≤0.016-0.03 | ||
| Imipenem | ≤0.016-0.03 | |||
| Meropenem | ≤0.016 | |||
| Ertapenem | ≤0.016 | |||
| Piperacillin-tazobactam | ≤0.125 | |||
| Amoxicillin-clavulanate | ≤0.125 | |||
| Ceftriaxone | ≤0.125 | |||
| Clindamycin | ≤0.016 | |||
| Metronidazole | ≤0.125-0.25 | |||
| Porphyromonas gingivalis (0/3) | Doripenem | ≤0.016 | ||
| Imipenem | ≤0.016 | |||
| Meropenem | ≤0.016 | |||
| Ertapenem | ≤0.016 | |||
| Piperacillin-tazobactam | ≤0.125 | |||
| Amoxicillin-clavulanate | ≤0.125 | |||
| Ceftriaxone | ≤0.125 | |||
| Clindamycin | ≤0.016 | |||
| Metronidazole | ≤0.125 | |||
| Porphyromonas levii (0/1) | Doripenem | 0.03 | ||
| Imipenem | ≤0.016 | |||
| Meropenem | ≤0.016 | |||
| Ertapenem | ≤0.016 | |||
| Piperacillin-tazobactam | ≤0.125 | |||
| Amoxicillin-clavulanate | ≤0.125 | |||
| Ceftriaxone | ≤0.125 | |||
| Clindamycin | ≤0.016 | |||
| Metronidazole | ≤0.125 | |||
| Fusobacterium nucleatum (2/20) | Doripenem | ≤0.016-0.03 | ≤0.016 | 0.03 |
| Imipenem | ≤0.016-0.06 | 0.03 | 0.03 | |
| Meropenem | ≤0.016-0.06 | ≤0.016 | 0.03 | |
| Ertapenem | ≤0.016-0.06 | ≤0.016 | 0.03 | |
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-0.5 | ≤0.125 | ≤0.125 | |
| Ceftriaxone | ≤0.125-0.5 | 0.25 | 0.5 | |
| Clindamycin | 0.03-0.125 | 0.06 | 0.125 | |
| Metronidazole | ≤0.125-0.5 | ≤0.125 | ≤0.125 | |
| Fusobacterium necrophorum (0/18) | Doripenem | ≤0.016-0.25 | ≤0.016 | 0.25 |
| Imipenem | ≤0.016-0.25 | ≤0.016 | 0.25 | |
| Meropenem | ≤0.016-0.125 | ≤0.016 | 0.125 | |
| Ertapenem | ≤0.016-0.125 | ≤0.016 | 0.125 | |
| Piperacillin-tazobactam | ≤0.125-0.5 | ≤0.125 | 0.5 | |
| Amoxicillin-clavulanate | ≤0.125-0.5 | ≤0.125 | 0.25 | |
| Ceftriaxone | ≤0.125 | ≤0.125 | ≤0.125 | |
| Clindamycin | 0.03-0.125 | 0.06 | 0.125 | |
| Metronidazole | ≤0.125-0.5 | ≤0.125 | 0.5 | |
| Fusobacterium varium (0/10) | Doripenem | 0.06-1 | 0.125 | 0.25 |
| Imipenem | 0.5-2 | 1 | 1 | |
| Meropenem | 0.06-0.25 | 0.125 | 0.25 | |
| Ertapenem | 0.06-0.125 | 0.06 | 0.125 | |
| Piperacillin-tazobactam | 2-32 | 8 | 8 | |
| Amoxicillin-clavulanate | 1-4 | 2 | 4 | |
| Ceftriaxone | ≤0.125-8 | 4 | 8 | |
| Clindamycin | 2->16 | 4 | 16 | |
| Metronidazole | ≤0.125-0.5 | 0.25 | 0.5 | |
| Fusobacterium mortiferum (2/10) | Doripenem | 0.125-1 | 0.25 | 1 |
| Imipenem | 0.25-1 | 1 | 1 | |
| Meropenem | 0.06-1 | 0.125 | 0.5 | |
| Ertapenem | 0.06-0.5 | 0.06 | 0.5 | |
| Piperacillin-tazobactam | ≤0.125-4 | 0.25 | 2 | |
| Amoxicillin-clavulanate | 0.5-32 | 1 | 16 | |
| Ceftriaxone | ≤0.125->128 | 128 | ≥128 | |
| Clindamycin | 0.06-8 | 0.06 | 0.25 | |
| Metronidazole | ≤0.125 | ≤0.125 | ≤0.125 | |
| Finegoldia magna (0/10) | Doripenem | 0.06-0.125 | 0.06 | 0.125 |
| Imipenem | 0.03-0.06 | 0.06 | 0.06 | |
| Meropenem | 0.06-0.125 | 0.06 | 0.125 | |
| Ertapenem | 0.03-0.06 | 0.06 | 0.06 | |
| Piperacillin-tazobactam | ≤0.125-0.25 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-0.5 | 0.25 | 0.25 | |
| Ceftriaxone | 1-8 | 8 | 8 | |
| Clindamycin | 0.125->16 | 2 | >16 | |
| Metronidazole | 0.25-1 | 0.5 | 1 | |
| Micromonas micros (0/10) | Doripenem | ≤0.016-0.125 | ≤0.016 | 0.06 |
| Imipenem | 0.03-0.125 | 0.03 | 0.06 | |
| Meropenem | 0.03-0.25 | 0.03 | 0.25 | |
| Ertapenem | ≤0.016-0.25 | 0.03 | 0.125 | |
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125-1 | ≤0.125 | 0.5 | |
| Ceftriaxone | ≤0.125-2 | ≤0.125 | 1 | |
| Clindamycin | 0.125-0.5 | 0.25 | 0.5 | |
| Metronidazole | ≤0.125-0.5 | 0.25 | 0.5 | |
| Peptostreptococcus anaerobius (0/10) | Doripenem | 0.125-2 | 0.25 | 2 |
| Imipenem | 0.06-2 | 0.06 | 1 | |
| Meropenem | 0.25-2 | 0.25 | 2 | |
| Ertapenem | 0.25-2 | 0.5 | 2 | |
| Piperacillin-tazobactam | 0.25-16 | 0.25 | 16 | |
| Amoxicillin-clavulanate | 0.25-32 | 0.5 | 32 | |
| Ceftriaxone | 0.5-16 | 0.5 | 16 | |
| Clindamycin | 0.03-0.5 | 0.03 | 0.25 | |
| Metronidazole | ≤0.125-1 | 0.5 | 1 | |
| Anaerococcus prevotii (0/4) | Doripenem | 0.03 | ||
| Imipenem | ≤0.016-0.03 | |||
| Meropenem | ≤0.016-0.03 | |||
| Ertapenem | ≤0.016-0.125 | |||
| Piperacillin-tazobactam | ≤0.125 | |||
| Amoxicillin-clavulanate | ≤0.125 | |||
| Ceftriaxone | ≤0.125-0.5 | |||
| Clindamycin | 0.125-0.5 | |||
| Metronidazole | 0.25-2 | |||
| Anaerococcus tetradius (0/6) | Doripenem | ≤0.016-0.06 | 0.03 | |
| Imipenem | ≤0.016-0.03 | 0.03 | ||
| Meropenem | 0.03-0.06 | 0.03 | ||
| Ertapenem | 0.06-0.125 | 0.06 | ||
| Piperacillin-tazobactam | ≤0.125 | ≤0.125 | ||
| Amoxicillin-clavulanate | ≤0.125 | ≤0.125 | ||
| Ceftriaxone | 0.25-0.5 | 0.25 | ||
| Clindamycin | 0.5-1 | 0.5 | ||
| Metronidazole | 1-4 | 2 | ||
| Peptoniphilus asaccharolyticus (0/10) | Doripenem | ≤0.016-0.125 | ≤0.016 | 0.03 |
| Imipenem | ≤0.016-0.06 | ≤0.016 | 0.03 | |
| Meropenem | ≤0.016-0.125 | ≤0.016 | 0.03 | |
| Ertapenem | ≤0.016-0.5 | ≤0.016 | 0.06 | |
| Piperacillin-tazobactam | ≤0.125-0.25 | ≤0.125 | ≤0.125 | |
| Amoxicillin-clavulanate | ≤0.125 | ≤0.125 | ≤0.125 | |
| Ceftriaxone | ≤0.125-1 | ≤0.125 | 1 | |
| Clindamycin | 0.06->16 | 0.06 | >16 | |
| Metronidazole | ≤0.125-2 | 1 | 1 | |
| Clostridium perfringens (0/30) | Doripenem | ≤0.016-0.06 | 0.03 | 0.03 |
| Imipenem | ≤0.016-0.125 | 0.06 | 0.125 | |
| Meropenem | ≤0.016-0.03 | ≤0.016 | ≤0.016 | |
| Ertapenem | ≤0.016-0.125 | 0.06 | 0.125 | |
| Piperacillin-tazobactam | ≤0.125-1 | 0.25 | 0.5 | |
| Amoxicillin-clavulanate | ≤0.125 | ≤0.125 | ≤0.125 | |
| Ceftriaxone | ≤0.125-4 | 2 | 4 | |
| Clindamycin | 0.06-4 | 1 | 2 | |
| Metronidazole | 0.5-2 | 1 | 2 | |
| Clostridium difficile (0/20) | Doripenem | 1-4 | 2 | 4 |
| Imipenem | 4-8 | 8 | 8 | |
| Meropenem | 1-4 | 2 | 4 | |
| Ertapenem | 4-8 | 4 | 8 | |
| Piperacillin-tazobactam | 8-32 | 16 | 32 | |
| Amoxicillin-clavulanate | 1-2 | 1 | 2 | |
| Ceftriaxone | 32-128 | 64 | 64 | |
| Clindamycin | 4->16 | 8 | >16 | |
| Metronidazole | 0.25-1 | 0.5 | 0.5 | |
| Clostridium innocuum (0/6) | Doripenem | 1 | 1 | |
| Imipenem | 1-2 | 1 | ||
| Meropenem | 1-2 | 2 | ||
| Ertapenem | 2-4 | 2 | ||
| Piperacillin-tazobactam | 1-2 | 1 | ||
| Amoxicillin-clavulanate | 0.25-0.5 | 0.5 | ||
| Ceftriaxone | 8-16 | 16 | ||
| Clindamycin | 0.5-2 | 1 | ||
| Metronidazole | 0.5-1 | 1 | ||
| Clostridium ramosum (0/5) | Doripenem | 0.5-1 | 0.5 | |
| Imipenem | 0.25-0.5 | 0.25 | ||
| Meropenem | 0.5-2 | 1 | ||
| Ertapenem | 1-2 | 1 | ||
| Piperacillin-tazobactam | ≤0.125-0.25 | 0.25 | ||
| Amoxicillin-clavulanate | ≤0.125 | ≤0.125 | ||
| Ceftriaxone | 0.25-0.5 | 0.5 | ||
| Clindamycin | 2-8 | 4 | ||
| Metronidazole | 0.5-2 | 2 | ||
| Clostridium bifermentans (0/4) | Doripenem | 0.06-0.125 | ||
| Imipenem | 0.25-0.5 | |||
| Meropenem | 0.125 | |||
| Ertapenem | 0.125 | |||
| Piperacillin-tazobactam | 0.5-1 | |||
| Amoxicillin-clavulanate | 0.25-2 | |||
| Ceftriaxone | 0.25 | |||
| Clindamycin | 0.06-0.25 | |||
| Metronidazole | 0.25-0.5 | |||
| Clostridium clostridioforme (0/2) | Doripenem | 1 | ||
| Imipenem | 2 | |||
| Meropenem | 1 | |||
| Ertapenem | 1-2 | |||
| Piperacillin-tazobactam | 16 | |||
| Amoxicillin-clavulanate | 1 | |||
| Ceftriaxone | 64 | |||
| Clindamycin | 0.06 | |||
| Metronidazole | ≤0.125-0.25 | |||
| Clostridium sordellii (0/2) | Doripenem | 0.03 | ||
| Imipenem | 0.125 | |||
| Meropenem | 0.06 | |||
| Ertapenem | 0.03 | |||
| Piperacillin-tazobactam | 0.25 | |||
| Amoxicillin-clavulanate | ≤0.125 | |||
| Ceftriaxone | ≤0.125-0.25 | |||
| Clindamycin | 1 | |||
| Metronidazole | 0.5-1 | |||
| Clostridium paraputrificum (0/1) | Doripenem | 0.25 | ||
| Imipenem | 0.5 | |||
| Meropenem | 0.25 | |||
| Ertapenem | 0.125 | |||
| Piperacillin-tazobactam | 0.25 | |||
| Amoxicillin-clavulanate | 0.5 | |||
| Ceftriaxone | 4 | |||
| Clindamycin | 8 | |||
| Metronidazole | 0.5 | |||
| Clostridium cadaveris (0/1) | Doripenem | 0.03 | ||
| Imipenem | 0.125 | |||
| Meropenem | 0.03 | |||
| Ertapenem | ≤0.016 | |||
| Piperacillin-tazobactam | 0.5 | |||
| Amoxicillin-clavulanate | ≤0.125 | |||
| Ceftriaxone | 4 | |||
| Clindamycin | 0.03 | |||
| Metronidazole | 0.25 | |||
The numbers of β-lactamase-positive strains/numbers of strains tested are indicated in parentheses.
Agar dilution MICs were determined according to current CLSI (previously NCCLS) methodology (14) with brucella blood agar plates with added laked sheep blood, hemin, and vitamin K. The inocula scraped from plates with approximately 1 × 105 CFU/spot were applied to plates by means of a Steers replicator, and plates were incubated for 48 h at 35°C inside an anaerobic glove box (Coy Laboratory Products, Grass Lake, MI). The MIC was read as the concentration where marked reduction occurred in the level of growth on the test plate compared to that on the anaerobic control plate. Standard quality control strains (14) were included with each run. Tazobactam was added to piperacillin at a fixed concentration of 4 μg/ml. β-Lactamase production was tested by the Cefinase disk method (Becton, Dickinson and Company, Sparks, MD).
For time-kill studies, a method devised in our laboratories was used (6, 9, 15, 16). Inocula were prepared inside the anaerobe chamber from brucella blood plates in tubes containing 5 ml prereduced brucella broth. A 100-μl aliquot of diluted inoculum with a final concentration of 106 to 107 CFU/ml was delivered into each vial containing 2.9 ml prereduced brucella broth with 5% laked horse blood cells, 5 μg of hemin/ml, 1 μg of vitamin K1/ml, and 1 ml of antibiotic dilution (prepared in prereduced brucella broth). All preparations and dilutions were prepared inside the chamber. Vials were removed from the chamber and incubated for 48 h in a shaking water bath at 35°C. For metronidazole, 200 μl of Oxyrase solution (Oxyrase, Inc., Mansfield, OH) was added. Antibiotic ranges at the MIC, 2× MIC, and 4× MIC were tested. One antibiotic-free growth control was used in each test. Viability counts were performed at 6 h, 12 h, 24 h, and 48 h, with plates being incubated for 48 h inside the chamber (6, 9, 15, 16).
The results of MIC testing can be seen in Table 1. Doripenem was potent (≤1 μg/ml) against all organism groups, except for some strains of Bacteroides fragilis, Bacteroides ovatus, Peptostreptococcus anaerobius, and Clostridium difficile, and had MICs similar to those of imipenem, meropenem, and ertapenem. Amoxicillin-clavulanate at the CLSI breakpoint was active against all groups except for some members of the B. fragilis group, Fusobacterium mortiferum, and P. anaerobius. Piperacillin-tazobactam was active against all but C. difficile and some Bacteroides vulgatus strains. Ceftriaxone was inactive against most β-lactamase-positive gram-negative rods, F. mortiferum, C. difficile, and a few other Clostridium spp. Clindamycin resistance was found among gram-negative and gram-positive strains (especially C. difficile). All strains were susceptible to metronidazole.
The MICs of the 14 strains determined by time-kill analyses are listed in Table 2. In all cases, agar dilution MICs did not differ by >1 dilution from MICs obtained by the inspection of macrodilution time-kill tests. Time-kill results are presented in Table 3 for all drug concentrations and all time periods tested. Four strains with piperacillin-tazobactam MICs of ≤0.004 μg/ml and four with ceftriaxone MICs of >32 μg/ml were not tested by time-kill methods. All four carbapenems were bactericidal (99.9% killing) at 2× MIC after 24 h against 10 strains and demonstrated 99% killing of all 14 strains at 2× MIC after 48 h. Doripenem and imipenem resulted in 99% killing of all strains at 48 h at the MIC and also demonstrated 90% killing of 12 strains at the MIC after 6 h. The three strains which were not killed by 99.9% by doripenem, imipenem, and ertapenem at 2× MIC after 48 h were one strain of Finegoldia magna and two of C. difficile; one additional strain of F. magna was not bactericidally inhibited by meropenem at 2× MIC after 48 h. However, doripenem and imipenem exhibited 99% killing of all 14 strains at the MIC after 48 h, and 90% killing was observed with all carbapenems at the MIC after 24 h. Carbapenem MICs for strains not bactericidally inhibited at 2× MIC after 48 h ranged from 0.03 to 0.125 μg/ml (F. magna) and 1 to 4 μg/ml (C. difficile).
TABLE 2.
MICs of all agents against the 14 strains tested by time-kill analyses
| Drug | MIC(s) (μg/ml)a for:
|
||||||
|---|---|---|---|---|---|---|---|
| Bacteroides fragilis | Bacteroides thetaiotaomicron | Prevotella intermedia | Finegoldia magna | Fusobacterium nucleatum | Clostridium perfringens | Clostridium difficile | |
| Doripenem | 0.25 | 0.25, 0.5 | 0.06, 0.125 | 0.06 | 0.016 | 0.03 | 1.0 |
| Imipenem | 0.06 | 0.25 | 0.03, 0.06 | 0.06, 0.125 | 0.016 | 0.06 | 4.0 |
| Ertapenem | 0.25 | 0.5, 1.0 | 0.03, 0.125 | 0.03, 0.06 | 0.008 | 0.125 | 4.0 |
| Meropenem | 0.125 | 0.25, 0.5 | 0.03, 0.25 | 0.06 | 0.008 | 0.03 | 1.0, 2.0 |
| Piperacillin-tazobactam | 0.125, 0.25 | 8.0, 16.0 | NTb | 0.125, 0.25 | NTb | 0.25 | 8.0 |
| Amoxicillin-clavulanate | 2.0 | 2.0 | 0.06, 0.25 | 0.25 | 0.06, 0.25 | 0.06, 0.125 | 0.5, 1.0 |
| Ceftriaxone | 0.25, 0.5 | NTc | 0.125, 8.0 | 8.0, 16.0 | 0.125, 0.25 | 2.0, 4.0 | NTc |
| Clindamycin | 0.5 | 2.0, 4.0 | 0.016 | 0.25, 4.0 | 0.06, 0.125 | 0.125, 2.0 | 8.0 |
| Metronidazole | 2.0 | 1.0 | 0.5, 1.0 | 1.0, 2.0 | 0.016, 0.25 | 2.0 | 0.5 |
Two strains of each species were tested. Intraspecies MICs which differed from one another are separated with a comma. NT, not tested.
This strain had an MIC of ≤0.004 μg/ml.
This strain had an MIC of >32.0 μg/ml.
TABLE 3.
Results of time-kill assays at the various time periods tested and at different drug concentrations
| Drug and concn | No. of strains killed by indicated %a at:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 h
|
12 h
|
24 h
|
48 h
|
|||||||||
| 90% | 99% | 99.9% | 90% | 99% | 99.9% | 90% | 99% | 99.9% | 90% | 99% | 99.9% | |
| Doripenem | ||||||||||||
| 4× MIC | 12 | 11 | 8 | 12 | 10 | 10 | 14 | 12 | 10 | 14 | 14 | 11 |
| 2× MIC | 12 | 10 | 8 | 12 | 10 | 10 | 14 | 12 | 10 | 14 | 14 | 11 |
| MIC | 12 | 10 | 8 | 12 | 10 | 8 | 14 | 11 | 9 | 14 | 14 | 9 |
| Imipenem | ||||||||||||
| 4× MIC | 12 | 10 | 9 | 13 | 10 | 9 | 14 | 13 | 10 | 14 | 14 | 11 |
| 2× MIC | 12 | 10 | 9 | 13 | 10 | 9 | 14 | 13 | 10 | 14 | 14 | 11 |
| MIC | 12 | 10 | 9 | 13 | 10 | 9 | 14 | 13 | 10 | 14 | 14 | 10 |
| Meropenem | ||||||||||||
| 4× MIC | 12 | 10 | 7 | 12 | 10 | 9 | 14 | 12 | 10 | 14 | 14 | 10 |
| 2× MIC | 12 | 10 | 7 | 12 | 10 | 9 | 14 | 12 | 10 | 14 | 14 | 10 |
| MIC | 12 | 9 | 3 | 12 | 10 | 7 | 14 | 11 | 6 | 13 | 10 | 5 |
| Ertapenem | ||||||||||||
| 4× MIC | 12 | 9 | 8 | 13 | 10 | 10 | 14 | 12 | 10 | 14 | 14 | 11 |
| 2× MIC | 12 | 9 | 7 | 13 | 10 | 9 | 14 | 12 | 10 | 14 | 14 | 11 |
| MIC | 12 | 9 | 6 | 13 | 10 | 7 | 14 | 12 | 9 | 12 | 10 | 7 |
| Piperacillin-tazobactamb | ||||||||||||
| 4× MIC | 8 | 6 | 3 | 8 | 7 | 5 | 10 | 8 | 6 | 10 | 10 | 6 |
| 2× MIC | 7 | 3 | 3 | 8 | 5 | 5 | 10 | 7 | 5 | 10 | 9 | 3 |
| MIC | 6 | 2 | 2 | 8 | 5 | 3 | 10 | 6 | 4 | 10 | 6 | 2 |
| Amoxicillin-clavulanate | ||||||||||||
| 4× MIC | 11 | 9 | 6 | 14 | 10 | 8 | 14 | 14 | 11 | 12 | 12 | 10 |
| 2× MIC | 11 | 9 | 6 | 14 | 10 | 8 | 14 | 13 | 10 | 11 | 11 | 9 |
| MIC | 11 | 8 | 5 | 13 | 10 | 7 | 14 | 13 | 9 | 10 | 9 | 6 |
| Ceftriaxonec | ||||||||||||
| 4× MIC | 8 | 7 | 4 | 9 | 8 | 7 | 10 | 10 | 8 | 9 | 9 | 7 |
| 2× MIC | 8 | 7 | 4 | 9 | 8 | 5 | 10 | 9 | 8 | 8 | 8 | 6 |
| MIC | 8 | 4 | 3 | 7 | 6 | 3 | 7 | 6 | 5 | 5 | 4 | 2 |
| Clindamycin | ||||||||||||
| 4× MIC | 10 | 3 | 0 | 12 | 5 | 2 | 14 | 14 | 7 | 14 | 14 | 12 |
| 2× MIC | 8 | 3 | 0 | 12 | 5 | 1 | 14 | 13 | 7 | 13 | 13 | 10 |
| MIC | 8 | 3 | 0 | 12 | 5 | 0 | 14 | 12 | 4 | 12 | 9 | 5 |
| Metronidazole | ||||||||||||
| 4× MIC | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| 2× MIC | 14 | 12 | 11 | 14 | 14 | 13 | 14 | 14 | 13 | 14 | 13 | 13 |
| MIC | 13 | 10 | 4 | 14 | 12 | 8 | 13 | 12 | 11 | 11 | 10 | 10 |
Killing by 99.9% indicates bactericidal activity.
Four strains with MICs of ≤0.004 μg/ml were not tested.
Four strains with MICs of >32 μg/ml were not tested.
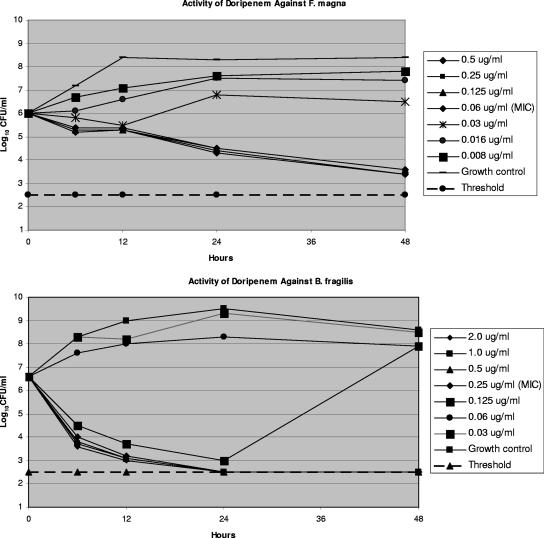
Metronidazole was bactericidal at 2× MIC against 13/14 strains after 24 h; the other drugs tested produced slower killing at all time periods. Doripenem and imipenem exhibited the best kill kinetics against all gram-negative rods tested, while doripenem and meropenem killed (99.9%) both C. perfringens strains at the MIC after 24 h and 48 h. Among all gram-positive strains, F. magna and C. difficile were killed more slowly by all carbapenems than other gram-positive and gram-negative organisms. The results of time-kill analyses at the MIC correlated well with the corresponding agar MIC. The kinetics of doripenem at 2× MIC after 48 h against one strain of B. fragilis, against which the drug was bactericidal, and one strain of F. magna, against which doripenem was bacteriostatic, are shown in Fig. 1.
FIG. 1.
Time-kill kinetics of doripenem against one strain of B. fragilis and one of F. magna. Viability counts are presented at various time periods between 0 and 48 h.
Wexler and coworkers, in a study of 364 anaerobes (17), showed that doripenem, ertapenem, imipenem, and meropenem were active against nearly all B. fragilis strains tested. Doripenem's activities were either comparable to or slightly less than those of imipenem and meropenem against most isolates but slightly more than those of ertapenem. Our results mirror preliminary results by other authors (5, 7, 8, 11, 12).
Against Streptococcus pneumoniae and Pseudomonas aeruginosa, Jones and coworkers (12) showed doripenem to be bactericidal at 8 h or later, with regrowth at 24 h in the case of P. aeruginosa. In our study, all four carbapenems gave excellent bactericidal activity, with doripenem and imipenem resulting in 99.9% killing of 11 strains at 2× MIC after 48 h; the 3 strains not killed comprised 1 F. magna strain and 2 C. difficile strains. C. difficile is known to be relatively insensitive to all β-lactams. We realize, of course, that relatively few species and strains were tested by time-kill analyses and that confirmation of our results must await reports from larger studies, which unfortunately appear rather sparsely in the literature.
In summary, doripenem yielded excellent potency and bactericidal activity, similar to those of other carbapenems, against most strains tested. The broad spectrum of activity of doripenem against Enterobacteriaceae and gram-negative nonfermenters points to a promising clinical future for this compound as a single therapy for mixed aerobic- and anaerobic-organism infections. Clinical studies, as well as pharmacokinetic/pharmacodynamic analyses, will be necessary to prove this hypothesis.
Acknowledgments
This study was supported by a grant from Johnson and Johnson Pharmaceutical Research and Development, Raritan, NJ.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Appelbaum, P. C. 1995. Quinolone activity against anaerobes: microbiological aspects. Drugs 49(Suppl. 2):76-80. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C., S. K. Spangler, and M. R. Jacobs. 1990. β-Lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole of 320 non-Bacteroides fragilis Bacteroides and 129 fusobacteria from 28 U.S. centers. Antimicrob. Agents Chemother. 34:1546-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelbaum, P. C., S. K. Spangler, and M. R. Jacobs. 1991. Susceptibilities of 394 Bacteroides fragilis, non-B. fragilis group Bacteroides species, and Fusobacterium species to newer antimicrobial agents. Antimicrob. Agents Chemother. 35:1214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelbaum, P. C., S. K. Spangler, and M. R. Jacobs. 1993. Susceptibility of 539 gram-positive and gram-negative anaerobes to new agents, including RP 59500, biapenem, trospectomycin and piperacillin/tazobactam. J. Antimicrob. Chemother. 32:223-231. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., E. Garber, Q. Zhao, Y. Ge, M. A. Wikler, K. Kanaga, and L. Saiman. 2005. In vitro activity of doripenem (S-4661) against multidrug-resistant gram-negative bacilli isolated from patients with cystic fibrosis. Antimicrob. Agents Chemother. 49:2510-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Credito, K. L., M. R. Jacobs, and P. C. Appelbaum. 2003. Time-kill studies of the antianaerobe activity of garenoxacin compared with those of nine other agents. Antimicrob. Agents Chemother. 47:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritsche, T., M. G. Stillwell, and R. N. Jones. 2005. Antimicrobial activity of doripenem (S-4661): a global surveillance report (2003). Diagn. Microbiol. Infect. Dis. 11:974-984. [DOI] [PubMed] [Google Scholar]
- 8.Ge, Y., M. A. Wikler, D. F. Sahm, R. S. Blosser-Middleton, and J. A. Karlowsky. 2004. In vitro antimicrobial activity of doripenem, a new carbapenem. Antimicrob. Agents Chemother. 48:1384-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoellman, D. B., L. M. Kelly, K. Credito, L. Anthony, L. M. Ednie, M. R. Jacobs, and P. C. Appelbaum. 2002. In vitro antianaerobic activity of ertapenem (MK-0826) compared to seven other compounds. Antimicrob. Agents Chemother. 46:220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs, M. R., S. K. Spangler, and P. C. Appelbaum. 1990. β-Lactamase production, β-lactam sensitivity and resistance to synergy with clavulanate of 737 Bacteroides fragilis group organisms from thirty-three US centres. J. Antimicrob. Chemother. 26:361-370. [DOI] [PubMed] [Google Scholar]
- 11.Jones, R. N., H. K. Huynh, and D. J. Biedenbach. 2004. Activities of doripenem (S-4661) against drug-resistant clinical pathogens. Antimicrob. Agents Chemother. 48:3136-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, R. N., H. K. Huynh, D. J. Biedenbach, T. R. Fritsche, and H. S. Sader. 2004. Doripenem (S-4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluation. J. Antimicrob. Chemother. 54:144-154. [DOI] [PubMed] [Google Scholar]
- 13.Jousimies-Somer, H. R., P. Summanen, D. M. Citron, E. J. Baron, H. M. Wexler, and S. M. Finegold. 2002. Wadsworth-KTL anaerobic bacteriology manual. Star Publishing Co., Belmont, CA.
- 14.National Committee for Clinical Laboratory Standards. 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard, 6th ed. NCCLS publication M11-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.Spangler, S. K., M. R. Jacobs, and P. C. Appelbaum. 1997. Bactericidal activity of DU-6859a compared to activities of three quinolones, three β-lactams, clindamycin, and metronidazole against anaerobes, as determined by time-kill methodology. Antimicrob. Agents Chemother. 41:847-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spangler, S. K., M. R. Jacobs, and P. C. Appelbaum. 1997. Time-kill study of the activity of trovafloxacin compared with ciprofloxacin, sparfloxacin, metronidazole, cefoxitin, piperacillin and piperacillin/tazobactam against six anaerobes. J. Antimicrob. Chemother. 39(Suppl. B):23-27. [DOI] [PubMed] [Google Scholar]
- 17.Wexler, H. M., A. E. Engel, D. Glass, and C. Li. 2005. In vitro activities of doripenem and comparator agents against 364 anaerobic clinical isolates. Antimicrob. Agents Chemother. 49:4413-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]