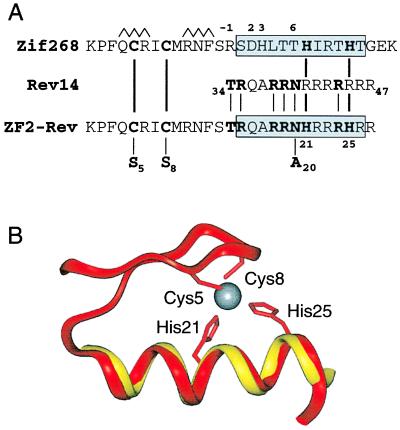
Figure 1.
Design of ZF-Rev peptides. (A) The sequence of Rev14 (24) was aligned with the α-helix of Zif268 zinc finger 2 (boxed) such that the histidines required for metal binding and the amino acids required for RRE recognition (shown in bold and indicated by lines) did not overlap. The numbering above the Zif268 α-helix indicates positions (−1, +2, +3, and +6, relative to the start of the helix) typically used for DNA binding, and the β-strands of the finger are indicated by open triangles. The putative α-helix in the hybrid ZF2-Rev is boxed. Positions of cysteine-to-serine mutations and an asparagine-to-alanine mutation are shown. A similar alignment was used to construct a fusion to Zif zinc finger 1 (ZF1-Rev; not shown). (B) Overlap between the α-helical segment of Zif finger 2 (magenta) (3) and residues 34–47 of the Rev peptide (yellow) (26). Backbone heavy atoms were superimposed from Ser-47 to Thr-58 of the finger and from Arg-35 to Arg-46 of the Rev peptide by using insight ii software (Biosym Technologies, San Diego) on a Silicon Graphics workstation. The rms deviation between all 36 superimposed atoms was 0.84 Å, and a similar rms deviation (0.93 Å) was obtained by using Zif finger 1 (not shown). The metal-coordinating cysteine and histidine residues and zinc ion in the finger are shown.