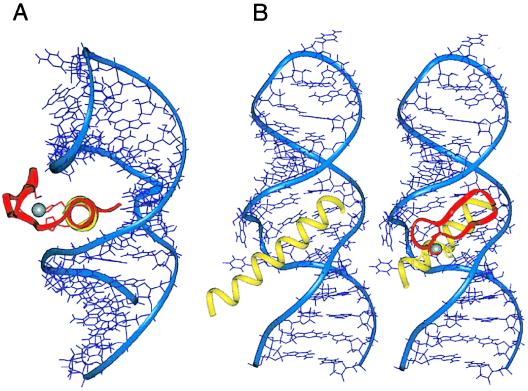
Figure 2.
(A) Proposed orientation of a zinc finger bound to RRE IIB. The α-helices of Rev (yellow) and Zif finger 2 (magenta) were superimposed and the aligned zinc finger was docked against the RNA. The view down the α-helical axes shows that the β-sheet portion of the finger emerges from the major groove and can accommodate the peptide backbone and side chains without steric clashes to the RNA. (B) Comparison of the Rev peptide- and ZF2-Rev-RRE complexes. The α-helix of Zif finger 2 was replaced by residues 34–47 of Rev, with two histidine substitutions (yellow). The β-sheet portion of the finger and the two cysteine ligands are shown in magenta and the zinc ion in white.