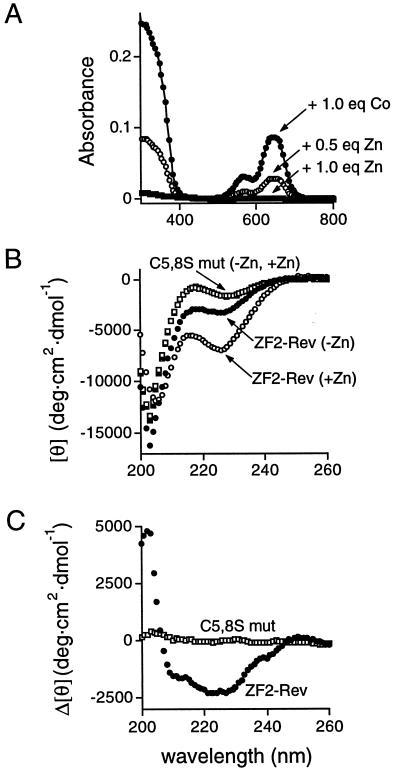
Figure 3.
Cobalt absorption spectroscopy and metal-dependent peptide folding. (A) Absorption spectra of ZF2-Rev (140 μM) in the presence of stoichiometric CoCl2 (●), or in the presence of 210 μM CoCl2 plus 70 μM (○) or 140 μM (■) ZnCl2 competitor. The spectra shown are characteristic of tetrahedral metal coordination in a monomeric zinc finger; red-shifted spectra have been observed with misfolded zinc finger dimers (29) and we observed similar shifts at low metal stoichiometries (data not shown), suggesting that incorrect complexes may be populated initially. (B) CD spectra of ZF2-Rev and a double-cysteine mutant (C5,8S): reduced ZF2-Rev in the absence of metal (●), ZF2-Rev in the presence of stoichiometric ZnCl2 (○), ZF2-Rev (C5,8S) in the absence of metal (■), and ZF2-Rev (C5,8S) in the presence of stoichiometric ZnCl2 (□). Spectra were recorded at 4°C with peptide concentrations of 30–60 μM. (C) Difference CD spectra of ZF2-Rev (●) and ZF2-Rev (C5,8S) (□), calculated by subtracting spectra in the absence of zinc from spectra in the presence of zinc (from B). The difference spectrum of ZF2-Rev shows two minima (at 208 and 222 nm) characteristic of α-helix formation whereas the spectrum of the cysteine mutant (see B) indicates little structure and no metal-dependent folding. Because ZF2-Rev showed some helix formation in the absence of zinc and had a higher helical content than ZF2-Rev (C5,8S), we performed several experiments to rule out the presence of adventitious zinc ions. We observed little change in the CD spectra by using buffers extensively treated with Chelex 100 chelating resin or in the presence of excess chelators (EDTA or 1,10-phenanthroline). Furthermore, careful titrations with cobalt and zinc showed no change in the visible absorption spectra after addition of stoichiometric CoCl2 and competition with stoichiometric amounts of ZnCl2, suggesting that little or no zinc was prebound to the peptide. In addition, 1D and 2D proton NMR spectra indicate that the peptide is unstructured in the absence of ZnCl2 and becomes ordered upon titration of stoichiometric ZnCl2 (W. Gmeiner, D.J.M., and A.D.F., unpublished data).