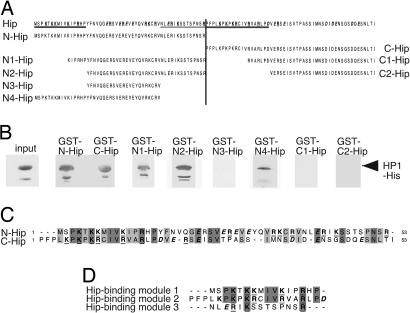
Fig. 4.
Hip contains three HP1-binding interfaces. (A) The Hip protein sequence (at the top) is unusually rich in charged amino acid residues, such as K, R, E, and D (bold type; positively and negatively charged amino acids are underlined and in italics, respectively). The N- and C-terminal fragments used for GST pull-down assays are indicated. (B) His-tagged full-length HP1 was used in GST pull-down assays with different Hip N-terminal (GST-N-Hip, GST-N1-Hip, GST-N2-Hip, GST-N3-Hip, and GST-N4-Hip) or C-terminal (GST-C-Hip, GST-C1-Hip, and GST-C2-Hip) fragments. Comparable amounts of protein were used in each experiment. Western blot analysis with anti-His tag antibody revealed that the Hip N- and C-terminal fragments are sufficient for HP1 binding. Fragments N1-Hip, N2-Hip, N4-Hip, but not N3-Hip, C1-Hip, or C2-Hip, interact with HP1. Three sequences that are necessary for HP1 binding are underlined in A. (C) Sequence comparison of the N- and C-terminal parts of Hip shows striking similarity. Identical residues are shaded in dark gray, chemically similar residues are indicated in light gray, and charged amino acids are in bold type. (D) Comparison of the three HP1-binding modules (underlined in A) reveals some similarity, especially in the position of charged residues, such as K and R.