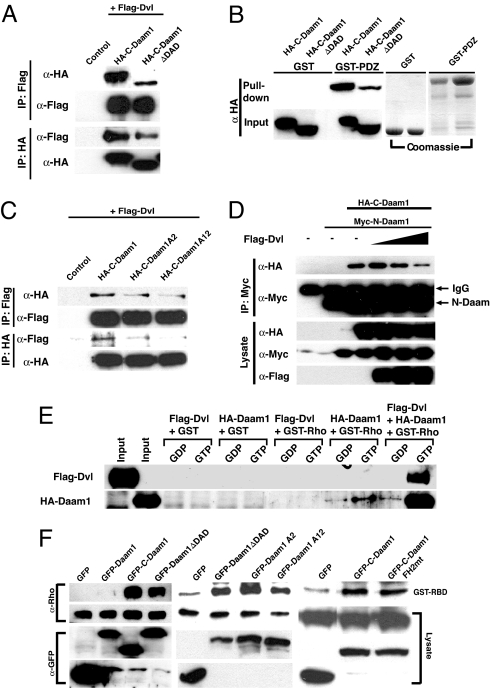
Fig. 2.
Dishevelled binds to the DAD of Daam1 and activates Daam1. (A) Coimmunoprecipitation assays reveal that Dvl binds to C-Daam but that its binding to C-DaamΔDAD is impaired. Plasmids encoding tagged Daam1 fragments were cotransfected into HEK293T cells, and cell lysates were immunoprecipitated (IP) with and immunoblotted with indicated Abs. (B) GST pull-down assays reveal that the PDZ domain of Dvl binds to C-Daam but that its binding to C-DaamΔDAD is reduced. (C) Single mutations within the DAD reduce whereas double mutations strongly impair interactions between Dvl and Daam1. (D) Dvl disrupts interactions between N-Daam and C-Daam. Increasing doses of Dvl were cotransfected with N-Daam and C-Daam into HEK293T cells, cell lysates were immunoprecipitated (IP) with indicated Abs, and precipitates were then immunoblotted with indicated Abs. (E) GST pull-down assays show that Daam1 binds to Rho-GTP with a higher preference over Rho-GDP but that the binding of Daam1 to Rho-GTP is amplified in the presence of Dvl. (F) Expression of Daam1 does not induce Rho activation in lysates from HEK293T cells, but C-Daam does. Removal of the DAD (DaamΔDAD) or mutations within the DAD of Daam1 induces Rho activation to levels similar to DaamΔDAD, and mutation within the FH2 domain of Daam1 that abolished the ability of Daam1 to induce stress fibers does not impair Rho activation.