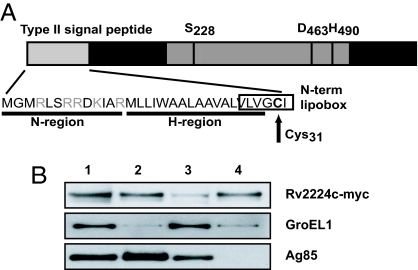
Fig. 1.
Rv2224c is a cell envelope-associated predicted hydrolase. (A) Rv2224c is predicted to be a lipoprotein with an N-terminal type II signal peptide. The N region has lysine and/or arginine, and the H region has hydrophobic residues. Cysteine at amino acid residue 31 within the conserved “lipobox” sequence is the putative lipoprotein attachment site, where diglyceride units are added by thioester linkage, followed by cleavage at a site immediately preceding the lipidated cysteine, which becomes the N terminus of the mature lipoprotein (6). Rv2224c contains the α/β hydrolase fold sequence and a catalytic triad typically present in serine proteases, esterases, and lipases, consisting of the catalytic nucleophile serine active site (S228), which associates with the proton carrier histidine (H490), and a charge relaying aspartic acid (D463). (B) Cell fractionation of M. bovis bacillus Calmette–Guérin-expressing myc-tagged Rv2224c and Western blots probed to detect the myc epitope, GroEL1, or Ag85 proteins. Lane 1, whole-cell extract; lane 2, cell wall; lane 3, cytoplasm; lane 4, cell membrane.