Abstract
Levels of thirst and ad libitum drinking decrease with advancing age, making older people vulnerable to dehydration. This study investigated age-related changes in brain responses to thirst and drinking in healthy men. Thirst was induced with hypertonic infusions (3.1 ml/kg 0.51M NaCl) in young (Y) and older (O) subjects. Regional cerebral blood flow (rCBF) was measured with positron emission tomography (PET). Thirst activations were identified by correlating rCBF with thirst ratings. Average rCBF was measured from regions of interest (ROI) corresponding to activation clusters in each group. The effects of drinking were examined by correlating volume of water drunk with changes in ROI rCBF from maximum thirst to postdrinking. There were increases in blood osmolality (Y, 2.8 ± 1.8%; O, 2.2 ± 1.4%) and thirst ratings (Y, 3.1 ± 2.1; O, 3.7 ± 2.8) from baseline to the end of the hypertonic infusion. Older subjects drank less water (1.9 ± 1.6 ml/kg) than younger subjects (3.9 ± 1.9 ml/kg). Thirst-related activation was evident in S1/M1, prefrontal cortex, anterior midcingulate cortex (aMCC), premotor cortex, and superior temporal gyrus in both groups. Postdrinking changes of rCBF in the aMCC correlated with drinking volumes in both groups. There was a greater reduction in aMCC rCBF relative to water drunk in the older group. Aging is associated with changes in satiation that militate against adequate hydration in response to hyperosmolarity, although it is unclear whether these alterations are due to changes in primary afferent inflow or higher cortical functioning.
Keywords: cingulate cortex, positron emission tomography
Under usual ambient conditions, volumes of water sufficient to maintain fluid balance are consumed in the routine of regular meals (1, 2). Heat or vigorous activity may cause dehydration, resulting in thirst and the desire to drink. Aging alters thirst and drinking responses, making older people vulnerable to body fluid imbalance (3). This study compared the pattern of brain responses in younger and older men during the development and the satiation of thirst. The aim of the study was to assess the role of cortical and subcortical processes in age-related changes of thirst and drinking.
There are two major physiological changes that cause the onset of thirst; increased blood/CSF osmolality and decreased volume of extracellular fluid. Studies differ on the effects of aging on the relations among blood tonicity, blood volume, and thirst. Increase of blood osmolality through the systemic infusion of hypertonic solutions has been reported to demonstrate either no age-related change in thirst ratings (4, 5) or decreased thirst responses in older men (6). Head-out water immersion drives water into the intrathoracic cavity, leads to increased blood volume and cardiac filling pressure in both young and older subjects, and has been used to assess the effects of blood volume changes on thirst ratings. Head-out immersion leads to a decrease in thirst in young subjects, but does not change thirst ratings in older subjects, suggesting that variation in blood volume has decreased impact on thirst with aging (7).
Irrespective of the factors giving rise to thirst, older people tend to drink less than their younger counterparts when dehydrated (8, 9). There has been considerable speculation about the effect of aging on the neural processes involved in the genesis and satiation of thirst. The maintenance of thirst responses to hypertonic infusion in older people would suggest that the neural responses to hyperosmolar stimuli are preserved with aging. Muted responses to hypovolemia could be explained by changes in baroreceptor function, although aging may impact on higher-order processing of signals generated by changes in blood volume. Slower, attenuated drinking responses to dehydration in older people could point toward age-related changes in neural processes that accompany satiation. In this respect, well documented age-related changes in sensory acuity for oropharyngeal stimuli (10) could contribute to altered responses to water consumption. That is, the gratification process involves a barrage of afferent inflow from mouth, pharynx, esophagus, and stomach in appropriate time sequence (11) and functional alteration in components of this may occur with age.
Age-related changes in the central processing of thirst and the satiation process could play a role in responses to dehydration in older people. We have used brain imaging techniques to measure responses to hypertonic infusions in humans and have identified widely distributed cortical and subcortical regions to be activated. Some of these may be neural correlates of consciousness of thirst. Others may reflect physiological mechanisms responding to change of extracellular tonicity, blood pressure, etc. that enhance activation of the neural systems that are the neural correlates of the subjective state termed thirst. The rise in osmolality and advent of thirst is associated with activations in the hypothalamus, thalamus, limbic cortex, somatosensory cortex, insula, and cerebellum (12–14). Of the regions so activated, the cingulate cortex appears to be particularly important, being rapidly responsive to satiation of thirst as indicated by neuroimaging changes. Our previous studies have identified regions of cingulate cortex in Brodmann Area 32 (BA32) (pregenual anterior cingulate cortex (pACC) and anterior midcingulate cortex (aMCC), which show a dramatic decrease in thirst-related activation immediately after drinking (13, 14). Reduced drinking in response to dehydration in older people could be related to dysfunction in the cortical regions contributing to the satiation process, of which the cingulate cortex is a likely candidate.
This study investigated the generation of thirst in older and younger subjects by infusion of hypertonic saline. Positron emission tomography (PET) was used to measure regional cerebral blood flow (rCBF) during the development and then the satiation of thirst. Thirst-related activations and responses to drinking were contrasted between the two groups to assess the impact of age on brain responses to the genesis and satiation of thirst.
Results
Physiological Measures and Verbal Ratings.
The sample consisted of 10 young men (Y) (mean age 23.7 ± 2.8 years) and 12 older men (O) (mean age 68.1 ± 3.4 years). The two groups had comparable mean body weights and resting heart rates (Table 1). A greater mean systolic blood pressure in the older group was approaching significance [t (20) = 2.0, P = 0.06] (Table 1). Both groups had low mean levels of thirst (Y, 1.1 ± 1.3; O, 0.8 ± 1.2) and dry mouth (Y, 1.3 ± 1.3; O, 0.7 ± 1.1) upon presentation to the scanning session. Ratings of thirst and dry mouth were highly correlated in both groups throughout the experiment (Y, r = 0.99; O, r = 0.97), and hereafter, reporting will be confined to thirst ratings only.
Table 1.
Demographic and baseline characteristics of the subject groups
| Young | Older | |
|---|---|---|
| Number | 10 | 12 |
| Age, yr | 23.7 (2.8) | 68.1 (3.4) |
| Weight, kg | 87.4 (10.8) | 85.1 (13.2) |
| Heart rate, BPM | 66.4 (8.7) | 64.2 (14.6) |
| Systole, mmHg | 129.9 (9.6) | 137.6 (15.1) |
| Diastole, mmHg | 68.9 (6.0) | 75.6 (9.2) |
| Thirst (0–10) | 1.1 (1.3) | 0.8 (1.2) |
| Dry mouth (0–10) | 1.3 (1.3) | 0.7 (1.1) |
Mean (±SD) values for demographic, physiological, and psychometric parameters for the young and older groups at baseline. BPM, beats per minute.
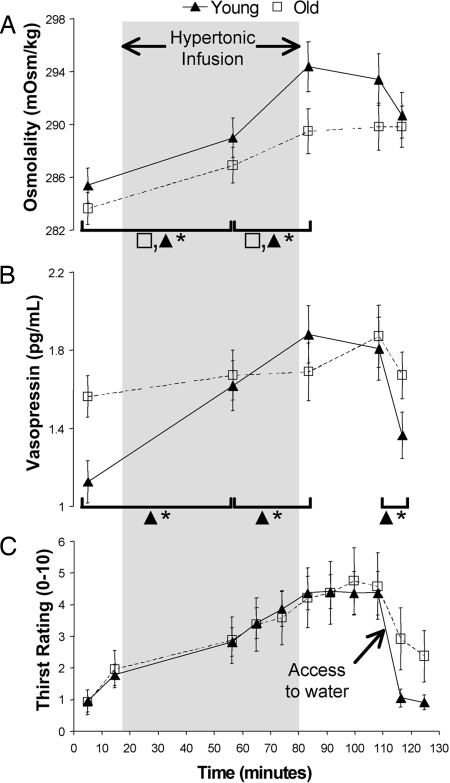
The two groups were infused with similar amounts of hypertonic saline relative to body weight (Y, 3.0 ± 0.4; O, 3.2 ± 0.3 ml/kg). Young and older subjects demonstrated increases in plasma Na [time effect F (4, 80) = 84.2, P < 0.001] that had a similar profile across the course of the protocol. A slightly lower mean plasma Na throughout the protocol in the older group failed to reach significance [F (1, 20) = 3.7, P < 0.07]. Pairwise comparisons revealed that plasma Na increased significantly from the baseline (139.3 ± 1.9 mmol/liter) to midinfusion (140.9 ± 2.2 mmol/liter)[t (21) = 7.8, P < 0.001], and from midinfusion to end-infusion (142.3 ± 2.5 mmol/liter)[t (21) = 6.8, P < 0.001]. No further significant changes occurred in plasma Na after the end of the infusion (predrink 142.7 ± 1.6 mmol/liter, postdrink 143 ± 1.8 mmol/liter). Changes in osmolality were similar to the changes in plasma Na. A significant time effect for osmolality [F (4, 80) = 24.3, P < 0.001] was due to increases from baseline to midinfusion [t (21) = 5.2, P < 0.001], and from midinfusion to end-infusion [t (21) = 7.1, P < 0.001] (Fig. 1A). There was no between-group difference for osmolality, nor any interaction between group and time. Absent data for one older subject and an extreme value for one younger subject (5.8 SDs greater than mean) necessitated exclusion of these subjects from analysis of plasma arginine vasopressin (AVP) levels. A significant interaction between time and group [F (4, 72) = 2.9, P < 0.05] for AVP measures was due to changes across time in the young group that did not manifest in the older subjects. In the young group, AVP was lower than the older group at baseline [t (20) = 2.9, P < 0.01], increased from baseline to midinfusion [t (8) = 4.1, P < 0.005], increased from midinfusion to end-infusion [t (8) = 2.6, P < 0.05], and decreased from postinfusion (maximum thirst) to postdrink [t (8) = 4.0, P < 0.005] (Fig. 1B). There were no significant differences between sequential measures of AVP in the older subjects.
Fig. 1.
Plots of physiological responses and verbal ratings to the infusion of hypertonic saline followed by drinking in young and older groups. (A) Osmolality increased throughout the infusion in both groups and remained elevated thereafter. (B) Levels of vasopressin showed significant increases throughout the infusion in the young group and did not change in the older group. Drinking was associated with a marked decrease in vasopressin levels in the young group, whereas the older group showed a small, nonsignificant downward trend. (C) Old and young groups experienced similar increases in thirst in association with the infusion and reported decreased levels of thirst after drinking. *, P < 0.05
Thirst ratings changed significantly across the duration of the protocol [time effect F (3, 60) = 28.1, P < 0.000] (Fig. 1C). The ratings of the two groups did not differ significantly, nor was there any interaction between time and group. Thirst ratings increased from baseline to midinfusion [t (21) = 6.1, P < 0.001] and increased further in the postinfusion period [t (21) = 4.6, P < 0.001]. A significant decrease in mean thirst ratings occurred after drinking [t (21) = 5.6, P < 0.001]. The difference between the groups in postdrink levels of thirst was not significant [t (20) = 1.6], consistent with the absence of an interaction term. The mean volume of water ingested ad libitum by the young group (3.9 ± 1.9 ml/kg) was more than twice the volume ingested by the older subjects (1.9 ± 1.6 ml/kg) [t (20) = 2.7, P < 0.05]. The amount of water ingested correlated with increases in thirst from baseline to the postinfusion period in both groups (Y, r = 0.68, P < 0.05; O, r = 0.83, P < 0.001). Drinking-related decreases in AVP levels did not correlate with the volume of water ingested in either group.
Imaging.
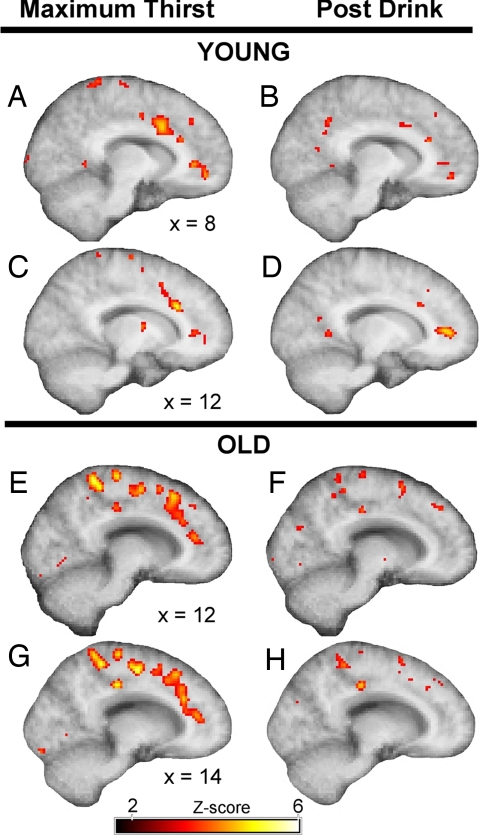
The contrast between maximum thirst and baseline revealed two loci of activation in the cingulate cortex in both groups: the aMCC [Y, (12, 24, 26); O, (12, 18, 48); Talairach coordinates] and the pACC (BA32) [Y, (8, 38, 2); O, (14, 36, 14)] (Fig. 2). The contrast between postdrink and baseline revealed activation in the pACC in the young group (12, 42, 6). Despite the proximity of the activation loci in aMCC in the two age groups, activation in the older group was predominantly in the dorsal aMCC (area 32′) (15), whereas activation in the younger group was primarily in areas 24a′/b′. Maximum thirst activation in the pACC was deeper in the right hemisphere in the older group (14, 36, 14) than in the younger group (8, 38, 2). The older group was notable for the presence of activations in the medial parietal and posterior cingulate cortices that was not apparent in the younger group.
Fig. 2.
Both age groups showed activation in the aMCC and pACC during maximum thirst. The loci of aMCC activations during maximum thirst were at (12, 24, 26) in the young group (A and C) and (12, 18, 48) in the older group (E and G). The postdrink period was notable for an absence of activation in the aMCC in the younger (B and D) and older (F and H) groups. Activation of the pACC during the postdrink period was absent in the older group (F and H), but was apparent at (12, 42, 6) in the younger subjects (D).
Ratings of thirst collected after each scan were correlated with rCBF measures for both groups. Thirst activation in the two groups included prefrontal, premotor, somatosensory, limbic, and temporal regions (Table 2). Cingulate cortex activations in the two age groups were deep in the right sulcus in the region of the aMCC, with a common location of activation at (12, 20, 28). Activation in the aMCC in the older group occupied a more rostral position (Fig. 3A). The older group had an additional thirst-related activation in the cingulate cortex, within the pACC. Thirst related activations were observed in the young group in the left hemisphere, in a region consistent with the central representation of the mouth and palate. The older group had bilateral activation in S1 that was located more dorsally. Both groups had activations in motor regions including the primary motor cortex, supplementary motor area, and premotor cortex. Activation in the cerebellum was confined to the older group. Additional activations in the young group included the inferior parietal lobule, precuneus, and inferior frontal gyrus bilaterally. Both groups had thirst-related activations in the superior temporal gyrus, in the left and right hemispheres, respectively. The older group also had activation in the middle temporal gyrus, middle frontal gyrus, and medial frontal gyrus that were not apparent in the younger group. The between group contrast of thirst activation did not reveal any significant differences between young and older subjects.
Table 2.
Thirst activations in the young and aged subject groups
| Region | Young |
Older |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BA | x | y | z | Z score | BA | x | y | z | Z score | |
| Activations | ||||||||||
| S1/M1 | 3/4 | −46 | −16 | 30 | 4.21 | 3/4 | −28 | −18 | 48 | 4.84 |
| 4 | −52 | −14 | 36 | 3.75 | 4 | 24 | −18 | 48 | 4.97 | |
| 2 | −54 | −28 | 36 | 3.58 | 3 | −20 | −22 | 46 | 4.74 | |
| Inf. parietal lob. | 40 | −36 | −38 | 48 | 4.81 | |||||
| SMA | 6 | −4 | 0 | 50 | 5.02 | 6 | 18 | 0 | 48 | 4.04 |
| 6 | 0 | −14 | 50 | 3.52 | 6 | −16 | −12 | 50 | 4.74 | |
| 6 | 0 | −16 | 70 | 4.43 | ||||||
| Premotor cortex | 6 | −30 | −8 | 40 | 4.94 | 6 | −32 | −10 | 36 | 5.29 |
| 6 | −32 | 2 | 36 | 3.90 | 6 | −14 | 14 | 48 | 4.77 | |
| Cingulate cortex | 24a′ | 10 | 12 | 32 | 5.82 | |||||
| 32 | 10 | 22 | 28 | 4.74 | 32 | 14 | 26 | 26 | 5.22 | |
| Med. F.G. | 8 | −12 | 28 | 42 | 4.38 | |||||
| Middle F.G. | 9 | 24 | 30 | 34 | 3.50 | |||||
| Inf. F.G. (orbital) | 47 | 52 | 32 | −2 | 4.84 | |||||
| 47 | −50 | 28 | −10 | 5.15 | ||||||
| Precuneus | 7 | −6 | −44 | 56 | 4.69 | |||||
| Middle Temp. G. | 39 | 34 | −72 | 12 | 5.42 | |||||
| Superior Temp. G. | 22 | −50 | 22 | −22 | 5.99 | 22 | 42 | 16 | −20 | 4.97 |
| Cerebellum | 2 | −68 | −14 | 4.22 | ||||||
| Deactivations | ||||||||||
| Superior F.G. | 10 | 20 | 62 | 18 | 5.41 | |||||
| 10 | 8 | 64 | −10 | 4.33 | ||||||
| Medial F.G. | 11 | −6 | 42 | −12 | 4.70 | 11 | 0 | 36 | −14 | 3.93 |
| 9 | −6 | 42 | 24 | 4.33 | ||||||
| Rectal G. | 11 | −10 | 22 | −20 | 4.49 | 11 | 6 | 28 | −20 | 4.26 |
| Superior Orb. G. | 19 | 34 | −72 | 24 | 4.98 | |||||
| Thalamus, Med. N. | −6 | −10 | 12 | 4.21 | ||||||
Thirst activations in the two groups were generated by correlating thirst ratings with rCBF measures. F., frontal; G., gyrus; I., inferior; lob., lobule; Med., medial; N., nucleus; Orb., orbital; S1/M1, primary sensory–motor cortex; Temp., temporal.
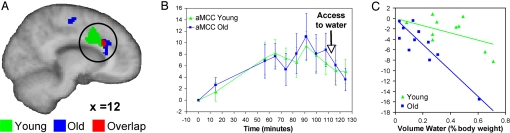
Fig. 3.
Activation was observed in the cingulate cortex in both groups. (A) The anterior midcingulate cortex (aMCC) (circled) was activated in both groups (Y: 10, 12, 32; O: 14, 26, 26). (B) The mean signal in the aMCC decreased after drinking. (C) The proportional change in aMCC signal change correlated with the amount of water drunk by subjects in both the young and older groups. The slope of the respective regression lines for the two groups indicates a greater level of signal change at lower levels of water drunk in the older group.
Regions of interest (ROI) were generated from thirst-related activation in the two age groups (see Methods). The effect of drinking was assessed by calculating a percentage signal change from the postinfusion period to the postdrink period for each subject. Drinking-related signal changes in ROIs were correlated with the volume of water ingested ad libitum. There were significant correlations in both groups, between volume of water ingested and drinking-related signal changes in the aMCC cluster (Y, r = −0.65, P < 0.05; O, r = −0.68, P < 0.05) (Fig. 3B). The respective regression equations for the young and old groups (Y, y = −6.8x; O, y = −25.4x) show a more pronounced signal change for lower volumes ingested in the older group (P < 0.05) (Fig. 3C). Signal changes in the S1/M1 cluster in the young group also correlated significantly with volume of water ingested (r = −0.72, P < 0.05), a relationship that was not evident in the older group.
Discussion
Older and younger subjects had similar increases in blood osmolality and experienced similar levels of thirst after systemic hypertonic infusion. The two age groups also exhibited similar patterns of rCBF associated with ratings of thirst. However, when given free access to water, older subjects drank much less than younger subjects. Thus, satiation of thirst, rather than its emergence and maintenance delineated the behavior of the young and older men in our study. Drinking behavior, measured by the volume of water drunk ad libitum was closely correlated to changes of rCBF in the aMCC in both age groups, but showed a greater degree of change at lower levels of water drunk in the older group.
In young people, the volume of water imbibed within a short time after a period of dehydration is usually sufficient to replenish body fluids (9). Older people tend to drink less water when dehydrated than their younger counterparts. This aged-related decrease in drinking has been reported after hypertonic infusions (6), fluid restriction (8), acutely after exercise (9), and in response to exercise–heat acclimation (16, 17). The disappearance of thirst after drinking usually occurs with a rapidity that precludes any contribution to the process of satiation from water absorption in the gut. The afferent inputs from oropharyngeal and gastric receptors stimulated by the ingestion of water are sufficient to abolish thirst despite the persistence of hypertonicity and decreased extracellular volume; the factors that give rise to and maintain thirst before drinking. The satiation of thirst depends on the integration of afferent inputs that accurately code the volume of water ingested (11, 18). It is not clear at what juncture age-related change intersects with the train of events that result in satiation, but two major possibilities arise. One possible mechanism is that gustatory responses and/or primary afferent mechanisms undergo age-related changes that degrade the accuracy of sensory signals representing the volume of water ingested in response to thirst. A second potential mechanism is that the cortical processes involved in the integration of input from osmoreceptors, baroreceptors, and gut and oropharangeal afferents are changed by aging. That is, the change is in the cortex—not the afferent inflow.
Many sensory modalities demonstrate functional deterioration with advancing age. Decrements in the sensations of smell and taste among older people are well established (10). Distension of the esophagus and gut and associated sensations have been investigated in groups ranging in age from 18 to 87 years, revealing an age-related decrease in pain thresholds and feelings of fullness despite unaltered gut compliance to pressure (19, 20). Cerebral evoked responses elicited by distension of the esophagus have a longer latency and smaller amplitude in older compared with younger subjects (21). In our experiment, rCBF levels in the S1 were associated with volume of water drunk in the young but did not exhibit a relationship in older subjects, pointing toward differences of primary afferent processes in the older group. However, to be compatible with a decrease in water drunk, a disturbance of afferent function would have to cause an overestimation of water ingested. It seems unlikely that loss of primary afferent function would lead to a decrease in drinking, particularly in light of the observation that elimination of vagal afferent fibers via capsaicin treatment leads to increased drinking in rats (22).
It is possible that different components of the intestinal tract express different types of age-related change. The presence or absence of aging effects on the innervation and function of the stomach could be critical for the satiation process in older people. At a structural level, the aging stomach is more notable for decreasing levels of sympathetic innervation than for loss of afferent fibers (23, 24). Motor responses in the stomach are likely to influence the passage of water through the gut, and consequently age associated sympathetic fiber loss could effect the accurate coding of water volume in the stomach. In an investigation of age-related changes in appetite, the volume of the distal stomach was significantly greater after water preloading in older subjects compared with younger subjects (25). Increased afferent input subsequent to excessive stomach distension in older people could lead to more rapid satiation of thirst after drinking.
The cingulate cortex is frequently activated in association with sensations arising from stimulation of interoceptors. Previous reports from this group have identified activations associated with thirst (13, 14, 26) and plasma Na (27) in two regions of the cingulate cortex: the pACC and the aMCC (summarized graphically in ref. 28). Contrasts between baseline scans and later scans in the study reported herein replicated our previous findings in that aMCC and pACC were activated during maximum thirst, and the activations disappeared in the postdrink period, with the exception of a cluster in the pACC for the postdrink contrast in the young group. The relationships of the respective thirst activations with ratings of thirst and drinking in the two age groups are compatible with the four-region neurobiological model advocated for the cingulate cortex. According to the four-region model, the anterior cingulate cortex [incorporating the pregenual (pACC) and the subgenual (sACC) divisions] is involved in the regulation of autonomic motor function and emotion, whereas the midcingulate cortex is ascribed with a role in premotor functions, particularly with regard to selection of behaviors associated with reward contingencies (15).
The pACC appeared to have distinct activations in the old and young in that it manifested only in the young group in the postdrink period. However, a test of between group differences in thirst-activations did not show age-related changes in this subdivision of the anterior cingulate cortex. In as much as subjective thirst ratings are an index of emotional resolve, the two age groups were also notable for an absence of differences. It contradistinction to the pACC, activations in the aMCC were of direct relevance to the major behavioral difference between the age groups—volume of water drunk ad libitum. The close association between volumes of water drunk and activation in the aMCC is consistent with a pivotal role for this region of the cingulate cortex in the regulation of behaviors leading to the satiation of thirst. It seems likely that age-related changes in the cortex alter aMCC function in that goal-directed behaviors engendered by thirst—the search for and drinking of water—fail to adequately resolve disturbances of fluid balance in older people.
The young and older subjects had similar physiological and subjective responses up to and including the period of maximum thirst. Thirst ratings were very similar for the two groups. Thirst activations generally followed a similar pattern in young and older subjects, although the loci of clusters varied within commonly activated anatomical regions but were not sufficiently divergent to constitute statistically significant age effects. The osmolality and plasma Na changed in predictable fashion in both groups, increasing during the course of the infusion and remaining elevated thereafter. The most striking difference between the two groups throughout the protocol was the disparity in AVP responses. Significant changes failed to materialize in the older group for successive comparisons of AVP levels, whereas the young subjects had a much lower baseline level, demonstrated a marked increase during the infusion, and a precipitate decline subsequent to drinking. The age-related attenuation of AVP responses to hypertonic infusion and drinking has precedent in other experimental manipulations of thirst (29). There is evidence from human and animal experimentation to suggest that afferent input from the oropharangeal region is particularly important for the inhibition of AVP release subsequent to drinking (30, 31). It is possible that age-related decreases in sensory function associated with stimulation of the oropharangeal region could play a part in the lack of AVP suppression postdrinking. This differential response could be in distinction to the process of satiation, which may depend more on afferent input from the stomach in older men.
Conclusion
Aging is associated with decreased levels of drinking subsequent to infusion of hypertonic saline. The satiation of thirst at lower levels of drinking in older men may be related to sensitized responses of the aMCC to afferent inflow from the oropharangeal and gastric regions. This age-related effect on satiation occurred despite very similar levels of thirst before drinking. The apparent failure of central processes contributing to satiation has important public health implications for older people in hot environments or undertaking exercise (34). Scheduled drinking may be a strategy to reduce the risk of dehydration in older people, although care should be exercised to avoid excessive water intake and the associated risks of cerebral swelling.
Methods
Subject Recruitment and Preliminary Procedures.
A group of 10 healthy younger subjects and a group of 12 healthy older subjects were recruited. Before being enlisted in the study, subjects were medically reviewed to exclude the presence of any disorders or medications likely to influence fluid balance or cerebral blood flow. Subjects were instructed to abstain from alcohol for 24 h before the PET scanning session. On the day of scanning, which occurred in the morning, subjects were instructed to have a light breakfast including 150 ml of either water or juice and no other beverages. The protocol for this study was approved by the Institutional Review Board of the University of Texas Health Sciences Center.
Images.
High-resolution anatomical images were acquired with a 1.9T Elscint/GE magnetic resonance scanner, using a T1 weighted 3D, gradient recalled echo sequence repetition time = 35 ms, echo time = 7 ms, flip angle = 60°, 1-mm2 sagittal slices, 1.3-mm slice thickness). PET images were acquired with an ECAT HR+ PET Scanner (CTI, Knoxville, TN) with 63 transverse slices of 2.5-mm thickness. For each scan, subjects received a 15-mCi bolus injection of H215O.
Protocol.
Subjects were positioned on the PET scanner table and fitted with a thermoplastic face mask to reduce head movement. Cannulae were inserted in veins in both arms for the infusion of hypertonic saline (left arm) and the administration of H215O doses and collection of blood samples (right arm). Ratings of thirst and dry mouth were collected after all scans by using a 10-cm visual analogue scale with the anchors no thirst, no dry mouth, and most intense thirst ever experienced, most intense dry mouth ever experienced. The first two scans were performed during rest, eyes closed. Hypertonic infusions (5% saline, 0.51 M NaCl) were used to produce a hyperosmolar stimulus for the promotion of thirst. Our group has used this strategy in previously reported studies, consistently achieving increases in plasma Na and thirst ratings (13, 14, 26–28). The infusions were commenced after the second scan and continued for an average duration of 68.5 min. Scans 3–6 were performed during the hypertonic infusion. Scans 7–9 were collected after the infusion at maximum thirst. Subjects were given free access to water in the period between scans 9 and 10 (average of 8.3 min), and then two postdrink scans were acquired. Water was accessed through a hose fitted with a valve that could be grasped between the teeth and opened with gentle jaw pressure. Five blood samples were collected at baseline, start and end of infusion, maximum thirst, and postdrink (scans 1, 3, 6, 9, and 10 respectively).
Analysis.
Physiological parameters, thirst ratings and dry mouth ratings were tested for the effects of time (scans 1–11), group (old and young), and the interaction of time and group with repeated measures ANOVA. Post hoc testing was undertaken to determine the nature of any significant multivariate effects.
PET and MR images were coregistered and spatially normalized to the Talairach and Tournoux atlas (32) by using an affine, nine-parameter transformation (33). Images were spatially smoothed and globally normalized by using SPM2 (Wellcome Department of Cognitive Neurology, London). Statistical parametric maps (SPM) of thirst responses were generated with two analyses. To allow qualitative comparisons with previous reports of cingulate activations associated with systemic hypertonic infusions, contrasts were made between maximum thirst and baseline and between postdrink and baseline in both groups. The second analysis involved the correlation of rCBF with thirst ratings for all scans within each group. A between-group contrast of thirst activations generated from the correlation analyses was performed after applying a mask to include voxels with a positive parameter estimate for either of the within-group correlation analyses. Significant clusters of activation were identified by using a cluster level threshold (pcorr < 0.05) and a single voxel inclusion threshold of Z > 2.33 (P < 0.01). Clusters of activation within each group were defined as regions of interest (ROI). Mean signal intensities from the ROI were extracted from each subject for the calculation of percentage changes during the course of the protocol. The mean signal change in ROIs between the maximum thirst (scans 8 and 9) and postdrink periods (scans 10 and 11) from each subject were correlated with volume of water drunk.
ACKNOWLEDGMENT.
We thank Prof. Brent Vogt for advice in identifying cingulate cortex regions. This work was supported by the Robert J., Jr., and Helen C. Kleberg Foundation, the G. Harold and Leila Y. Mathers Charitable Foundation, the Brown Foundation, the Search Foundation, and the National Health and Medical Research Countil (NHMRC) of Australia. G.F.E. is a NHMRC Research Fellow (Grant 400317).
Footnotes
The authors declare no conflict of interest.
References
- 1.de Castro JM. Physiol Behav. 1988;43:705–714. doi: 10.1016/0031-9384(88)90367-8. [DOI] [PubMed] [Google Scholar]
- 2.Phillips PA, Rolls BJ, Ledingham JG, Morton JJ. Physiol Behav. 1984;33:357–363. doi: 10.1016/0031-9384(84)90154-9. [DOI] [PubMed] [Google Scholar]
- 3.Kenney WL, Chiu P. Med Sci Sports Exerc. 2001;33:1524–1532. doi: 10.1097/00005768-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Davies I, O'Neill PA, McLean KA, Catania J, Bennett D. Age Aging. 1995;24:151–159. doi: 10.1093/ageing/24.2.151. [DOI] [PubMed] [Google Scholar]
- 5.Stachenfeld NS, Mack GW, Takamata A, DiPietro L, Nadel ER. Am J Physiol. 1996;271:R757–R765. doi: 10.1152/ajpregu.1996.271.3.R757. [DOI] [PubMed] [Google Scholar]
- 6.Phillips PA, Bretherton M, Johnston CI, Gray L. Am J Physiol. 1991;261:R166–R171. doi: 10.1152/ajpregu.1991.261.1.R166. [DOI] [PubMed] [Google Scholar]
- 7.Stachenfeld NS, DiPietro L, Nadel ER, Mack GW. Am J Physiol. 1997;272:R148–R157. doi: 10.1152/ajpregu.1997.272.1.R148. [DOI] [PubMed] [Google Scholar]
- 8.Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ, Crowe MJ, Wollner L. N Engl J Med. 1984;311:753–759. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 9.Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. J Appl Physiol. 1994;76:1615–1623. doi: 10.1152/jappl.1994.76.4.1615. [DOI] [PubMed] [Google Scholar]
- 10.Schiffman SS. J Am Med Assoc. 1997;278:1357–1362. [Google Scholar]
- 11.Denton D. The Hunger for Salt. Berlin: Springer; 1982. [Google Scholar]
- 12.Parsons LM, Denton D, Egan G, McKinley M, Shade R, Lancaster J, Fox PT. Proc Natl Acad Sci USA. 2000;97:2332–2336. doi: 10.1073/pnas.040555497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinly M, Lancaster J, Fox P. Proc Natl Acad Sci USA. 1999;96:5304–5309. doi: 10.1073/pnas.96.9.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan G, Silk T, Zamarripa F, Williams J, Federico P, Cunnington R, Carabott L, Blair-West J, Shade R, McKinley M. Proc Natl Acad Sci USA. 2003;100:15241–6. doi: 10.1073/pnas.2136650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogt BA, Hof PR, Vogt LJ. In: The Human Nervous System. Paxinos G, Mai JK, editors. San Diego: Elsevier; 2004. [Google Scholar]
- 16.Takamata A, Ito T, Yaegashi K, Takamiya H, Maegawa Y, Itoh T, Greenleaf JE, Morimoto T. Am J Physiol. 1999;277:R1041–R1050. doi: 10.1152/ajpregu.1999.277.4.R1041. [DOI] [PubMed] [Google Scholar]
- 17.Zappe DH, Bell GW, Swartzentruber H, Wideman RF, Kenney WL. Am J Physiol. 1996;270:R71–R79. doi: 10.1152/ajpregu.1996.270.1.R71. [DOI] [PubMed] [Google Scholar]
- 18.Bernard C. Lecons de Physiologie Experimentale Applique à la Médecine Faites au College de France. Paris: Balliere; 1856. [Google Scholar]
- 19.Lasch H, Castell DO, Castell JA. Am J Physiol. 1997;272:G1–G3. doi: 10.1152/ajpgi.1997.272.1.G1. [DOI] [PubMed] [Google Scholar]
- 20.Rayner CK, MacIntosh CG, Chapman IM, Morley JE, Horowitz M. Scand J Gastroenterol. 2000;35:1041–1047. doi: 10.1080/003655200451153. [DOI] [PubMed] [Google Scholar]
- 21.Weusten BL, Lam HG, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. Eur J Clin Invest. 1994;24:627–631. doi: 10.1111/j.1365-2362.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 22.Curtis KS, Stricker EM. Am J Physiol. 1997;272:R704–R709. doi: 10.1152/ajpregu.1997.272.2.R704. [DOI] [PubMed] [Google Scholar]
- 23.Phillips RJ, Powley TL. J Comp Neurol. 2001;434:358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- 24.Phillips RJ, Rhodes BS, Powley TL. Anat Embryol (Berlin) 2006;211:673–683. doi: 10.1007/s00429-006-0123-z. [DOI] [PubMed] [Google Scholar]
- 25.Sturm K, Parker B, Wishart J, Feinle-Bisset C, Jones KL, Chapman I, Horowitz M. Am J Clin Nutr. 2004;80:656–667. doi: 10.1093/ajcn/80.3.656. [DOI] [PubMed] [Google Scholar]
- 26.Farrell MJ, Egan GF, Zamarripa F, Shade R, Blair-West J, Fox P, Denton DA. Proc Natl Acad Sci USA. 2006;103:2416–2421. doi: 10.1073/pnas.0511019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P. Proc Natl Acad Sci USA. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, Denton D. Proc Natl Acad Sci USA. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips PA, Bretherton M, Risvanis J, Casley D, Johnston C, Gray L. Am J Physiol. 1993;264:R877–R881. doi: 10.1152/ajpregu.1993.264.5.R877. [DOI] [PubMed] [Google Scholar]
- 30.Blair-West JR, Gibson AP, Woods RL, Brook AH. Am J Physiol. 1985;248:R68–R71. doi: 10.1152/ajpregu.1985.248.1.R68. [DOI] [PubMed] [Google Scholar]
- 31.Figaro MK, Mack GW. Am J Physiol. 1997;272:R1740–R1746. doi: 10.1152/ajpregu.1997.272.6.R1740. [DOI] [PubMed] [Google Scholar]
- 32.Talairach P, Tournoux J. A Stereotactive Coplanar Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- 33.Lancaster J, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox P. Hum Brain Mapp. 1995;3:209–223. [Google Scholar]
- 34.Vandentorren S, Suzan F, Medina S, Pascal M, Maulpoix A, Cohen JC, Ledrans M. Am J Public Health. 2004;94:1518–1520. doi: 10.2105/ajph.94.9.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]