Abstract
In western Europe, the Middle to Upper Paleolithic (M/UP) transition, dated between ≈35,000 and ≈40,000 radiocarbon years, corresponded to a period of major human biological and cultural changes. However, information on human population densities is scarce for that period. New faunal data from the high-resolution record of Saint-Césaire, France, indicate an episode of significant climatic deterioration during the early Upper Paleolithic (EUP), which also was associated with a reduction in mammalian species diversity. High correlations between ethnographic data and mammalian species diversity suggest that this shift decreased human population densities. Reliance on reindeer (Rangifer tarandus), a highly fluctuating resource, would also have promoted declines in human population densities. These data suggest that the EUP represented for humans a period of significant niche contraction in western Europe. In this context, the possibility that a modern human expansion occurred in this region seems low. Instead, it is suggested that population bottlenecks, genetic drift, and gene flow prevailed over human population replacement as mechanisms of evolution in humans during the EUP.
Keywords: archaeology, Rangifer, Middle to Upper Paleolithic transition, modern human origins, Neandertals
In western Europe, significant changes occurred during the Middle to Upper Paleolithic (M/UP) transition, including a widespread adoption of blade-based toolkits, the emergence of artistic behavior, and a shift toward modern human anatomical features. Focusing on population size and density, two key aspects of human evolution (1) might help in understanding the nature of some of these changes. However, little is known about population dynamics of human foragers before the Holocene (2, 3). Because forager density seems to be affected by climate (4), investigating the impact of climate change on human demography may yield insights into the evolution of human populations during the M/UP transition.
Ethnographically, human forager densities were particularly low in high latitudes, a pattern attributed to low plant and mammal species diversity and high fluctuations in ungulate productivity (4, 5). Human densities generally were higher in more temperate environments where mammal species are more diverse (6). In this article, the potential relationship between terrestrial mammal species diversity and human population density is scrutinized. The correlations obtained then are used to investigate the demographic implications for human groups of shifts in terrestrial mammal species diversity in the M/UP transition record. However, it should be noted that species diversity is only one of many possible parameters affecting human populations. Nevertheless, this parameter seems to provide a useful proxy for assessing changes in human densities.
Ecologists have long noted that species diversity of terrestrial mammals decreases with latitude and tends to be highest in the tropics, intermediate in temperate habitats, and lowest in the Arctic (7). Most of the changes in latitudinal gradients would be explained by correlates of temperature (e.g., annual minimum temperature, frost-free season length), moisture (e.g., evapotranspiration), and elevation (8, 9). Temporally, shifts in local climatic conditions can have effects similar to latitudinal gradients by increasing or decreasing species diversity (10).
In western Europe, Middle Paleolithic and early Upper Paleolithic (EUP) foragers depended mostly on terrestrial mammals for subsistence, particularly moderate (goat-sized) to large (bison-sized) ungulates. Marine mammals and lacustrine resources seem to have played a marginal role in the diet of these human populations (11) and, as a result, are not considered further here. North of the Pyrenees and the Alps, archaeofaunas dated to the M/UP transition generally are dominated by five species: aurochs (Bos primigenius), steppe bison (Bison priscus), horse (Equus caballus), red deer (Cervus elaphus), and reindeer (Rangifer tarandus). Paleoenvironmental reconstructions suggest that aurochs and red deer were most abundant in temperate woodlands and grasslands, whereas steppe bison and horses preferred temperate and cold steppe ecosystems (12). Today, these biomes are, by European standards, moderately rich in species (9). In contrast, reindeer would have been strongly associated with the boreal forest and the arctic tundra (12), two biomes with low species diversity. Therefore, temporal changes in the relative abundance of ungulate species may signal shifts in range occupancy and an associated reduction in species diversity that might have affected human populations. Here, ethnographic and wildlife data are used to predict how variations in terrestrial mammal species diversity may affect human population densities. These findings then are applied to the faunal sequence from Saint-Césaire, a M/UP transitional site in western France.
Mammalian Species Diversity Versus Forager Densities
Density data were compiled for 27 ethnographically documented forager populations from North America and compared with the total number of terrestrial mammalian species present historically within their respective territories (Table 1). Only Plains, Subarctic, and Arctic foragers were incorporated into the sample, to enhance comparability with Late Pleistocene ecosystems of western Europe (for a fuller discussion of the samples, see Materials and Methods).
Table 1.
Human densities and diversity of native mammalian species associated with 27 hunter–gatherer groups from North America
| Area | Group | Density, n/100 km2 | Species diversity | Location | Latitude N | Longitude W |
|---|---|---|---|---|---|---|
| Arctic | 1. Mountain people | 1.4 | 29 | Alaska | 67°30′ | 154°45′ |
| 2. Asiaqmiut | 1.0 | 18 | Nunavut | 62° | 99°45′ | |
| 3. Patliqmiut | 1.3 | 18 | Nunavut | 62° | 95° | |
| Subarctic | 4. Naskapi | 0.4 | 29 | Québec | 54° | 69° |
| 5. Chipewyan | 0.4 | 31 | Manitoba | 60° | 99°45′ | |
| 6. Attawapiskat Cree | 1.4 | 34 | Ontario | 52°30′ | 83°30′ | |
| 7. Dogrib | 0.6 | 31 | NW Territories | 64° | 116° | |
| 8. Hare | 0.5 | 27 | NW Territories | 66°45′ | 126° | |
| 9. Kolchan | 0.5 | 34 | Alaska | 62°30′ | 154°30′ | |
| 10. Slave | 1.4 | 39 | NW Territories | 60°30′ | 120° | |
| 11. Nabesna | 0.6 | 35 | Alaska | 62°30′ | 141° | |
| 12. Sekani | 1.0 | 46 | British Columbia | 56°30′ | 125°15′ | |
| 13. Yellowknife | 0.2 | 23 | NW Territories | 64°15′ | 111°45′ | |
| 14. Grand Lake Victoria Cree | 0.7 | 41 | Québec | 48°30′ | 77°45′ | |
| 15. Tahltan | 1.1 | 46 | British Columbia | 57°30′ | 130° | |
| 16. Beaver | 0.5 | 45 | Alberta | 57°15′ | 119° | |
| 17. Waswanipi Cree | 0.5 | 37 | Québec | 49°30′ | 76°30′ | |
| 18. Micmac | 2.3 | 40 | New Brunswick | 47° | 66°30′ | |
| Plains | 19. Blackfoot | 4.3 | 40 | Alberta | 51° | 112°30′ |
| 20. Plains Cree | 1.9 | 46 | Saskatchewan | 52° | 107° | |
| 21. Assiniboine | 5.8 | 44 | Saskatchewan | 50° | 105° | |
| 22. Crow | 2.6 | 60 | Montana | 45° | 108° | |
| 23. Arapaho | 3.0 | 76 | Colorado | 40° | 105° | |
| 24. Cheyenne | 3.0 | 66 | Wyoming | 42°30′ | 105°30′ | |
| 25. Kiowa-Apache | 1.4 | 48 | Oklahoma | 36° | 99° | |
| 26. Comanche | 5.0 | 52 | Texas | 33° | 100° | |
| 27. Kiowa | 1.4 | 57 | Oklahoma | 36°30′ | 102° |
Density data from Kelly (6) and Burch (5). The mid-value was adopted for Dogrib density. Numbers of native mammalian taxa were compiled by using the interactive maps of the Smithsonian Museum of Natural History web site on North American mammals (www.mnh.si.edu/mna//main.cfm). Coordinates corresponding to the center of the historical range occupied by each hunter–gatherer group were used to calculate species diversity as assessed based on maps in Sturtevant (46). Except for bison (added to the species list for nos. 16 and 19–27), no attempt was made to correct for recent changes in species distribution.
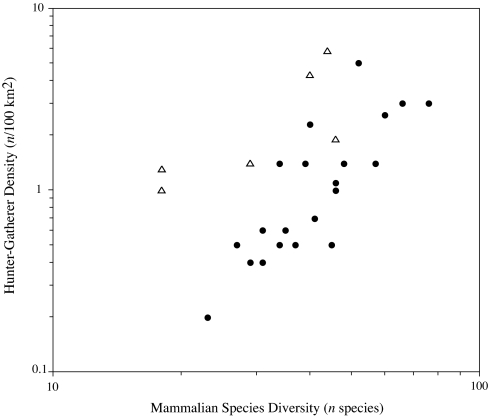
A positive correlation (rs = 0.62, P < 0.002) was found between forager density and mammalian species diversity in North America (Fig. 1). However, six human groups from cold, open country ecosystems seem to belong to a cluster distinct from the one formed by 21 forested northern and temperate grassland groups. The reason for this separation is not clear, but it may have to do with a latitudinal gradient in ungulate patchiness in open versus forested environments. Considering these two data sets separately increases the correlations (forested northern and temperate grassland groups: rs = 0.82, P < 0.001; cold, open country groups: rs = 0.81, P < 0.07). Importantly, the same relationship is found in both clusters: population densities decline with decreasing diversity of terrestrial mammalian species. Although these correlations do not provide proof of causality—they simply may reflect an underlying relationship driven by an as-yet-unidentified parameter (e.g., total animal biomass or total ungulate biomass)—they suggest that mammalian species diversity can be used to predict forager density, an approach compatible with the critical role played by terrestrial mammals in the diet of these human groups. As a result of the similarity of the compared ecosystems, it is reasonable to assume that shifts in terrestrial mammalian species diversity during the M/UP transition in western Europe would have been accompanied by changes in human population densities.
Fig. 1.
Relationship between hunter–gatherer density and mammalian species diversity (log-log scale). Triangles are cold, open-country human groups (Table 1, nos. 1–3 and 19–21), and solid circles are forested northern and temperate grassland human groups (Table 1, nos. 4–18 and 22–27).
Rangifer Fluctuations in Abundance and Human Demography
Historically, humans that were heavily dependent on wild Rangifer had low population densities and appear to have experienced demographic fluctuations of higher amplitude compared with populations with more diverse diets (4, 5).
The tendency of Rangifer populations to experience sporadic fluctuations of abundance, sometimes with rough patterns of periodicity, is known (13). For example, after a peak at the end of the 19th century, the George River herd (Québec, Canada) declined dramatically in the early 20th century and remained low until the 1960s, when it was estimated at ≈4,700 individuals and considered on the verge of extinction. Yet this population reached a new estimated peak of ≈776,000 caribou in 1993. In 2001, the estimate had fallen to ≈385,000 caribou, suggesting that the population had entered a new declining phase (14, 15). In this case, the amplitude of the fluctuation is 165:1.
Fluctuations of Rangifer abundance are extremely variable, with durations between highs and lows ranging from only decades to slightly more than a century. Some Rangifer populations in southwestern Alaska exhibit a 40- to 50-year periodicity (16), whereas in Greenland some Rangifer populations have a periodicity of 65–115 years (17). Peary caribou in the Canadian High Arctic have exhibited a relatively short 20-year periodicity (18). These fluctuations in abundance have been observed in the absence of human and carnivore predation and most commonly are attributed to overgrazing or to extremely unfavorable snow and ice conditions (13, 19).
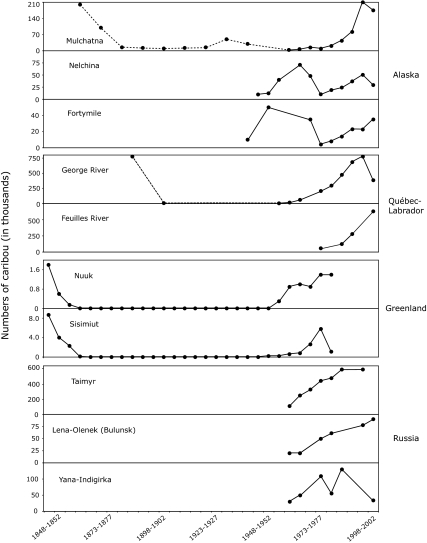
Another characteristic of several, but not all, Rangifer populations is that their lows tend to be synchronized at a regional scale, a phenomenon possibly mediated by large-scale climate systems such as the North Atlantic oscillation (20). These climate systems may, for example, result in prolonged severe snow and ice conditions over several consecutive years, causing catastrophic mortality. According to Fig. 2, the 1960s and 1970s corresponded to low numbers in several Rangifer populations of Alaska and Québec-Labrador, whereas the 1860–1950 period points to generally low caribou abundance in western Greenland, although the fit between regional populations is not always perfect. Little comparable data exist for Eurasia. Trends suggest that reindeer numbers might have been low during the 1950s in northern Russia, although the time series are often short and confounded by human intervention on wild populations (via reindeer breeding and extensive hunting) and conflicts in population size estimates (21). Regional synchrony in population lows is likely to have a negative impact on human densities by decreasing opportunities to secure food in times of crisis from relatives and friends established in other communities (the “safety net”) and by reducing the probability of finding higher Rangifer density in adjacent areas.
Fig. 2.
Temporal fluctuations of Rangifer populations in four regions: Alaska (data from refs. 16, 47, and 48), Québec-Labrador (data from refs. 14, 15, and 49), western Greenland (data from ref. 17), and northern Russia [data from refs. 21 (pp. 44 and 66) and 50 (pp. 79–83)]. All values connected by a solid line are based on surveys, except for Greenland (caribou skin trade counts before 1950, caribou kills after 1950). Values connected by a dashed line are extrapolations based on historical data. The 1935 value (500,000) for the Fortymile herd was not included here because it is probably an overestimation. When multiple values were available for a specific time interval, the one nearest to the middle of the interval was adopted.
Recent studies also suggest that fluctuations in Rangifer abundance increase with latitude, a pattern attributed to a south-to-north weakening of density dependence and to the increasing effect of stochastic abiotic factors along the same gradient (18, 20). Therefore, under severe climatic conditions, Rangifer-dependent foragers would have been more susceptible to demographic fluctuations compared with Rangifer-dependent foragers living in more permissive environments.
In summary, three aspects of Rangifer population dynamics may influence human population densities: (i) the high amplitude of the Rangifer fluctuations in abundance, (ii) the tendency for some Rangifer populations to decline synchronously at a regional scale, and (iii) the trend toward more frequent fluctuations of greater magnitude in Rangifer abundance with deteriorating climatic conditions and/or increasing latitude. How do these findings fit into our understanding of human demography during the late Pleistocene?
The Saint-Césaire Faunal Record
In 1979, a Neanderthal skeleton was found at the base of a limestone cliff at Saint-Césaire in Charente-Maritime, France, associated with Châtelperronian artifacts, an EUP industry then attributed to modern humans (22). This finding had important repercussions because it indicated that Neanderthals were involved in the emergence of the Upper Paleolithic.
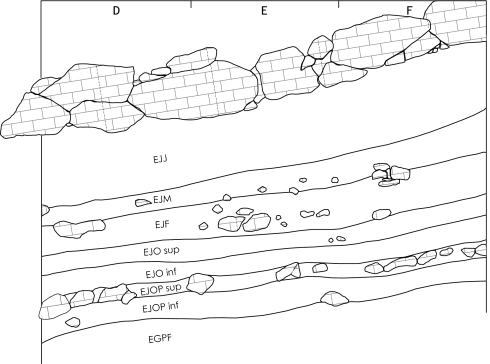
Further work at Saint-Césaire revealed a high-resolution stratigraphy covering the full span of the M/UP transition. Fifteen occupations, thermoluminescence-dated between 30 and 43 kyBP (23), along with several thousand artifacts and animal remains were uncovered during the excavations (24). In this sequence (Fig. 3), levels EGPF through EJJ are particularly important because they document the M/UP boundary. Level EGPF corresponds to a Denticulate Mousterian occupation, EJOP sup to a Châtelperronian occupation, EJO sup to a Proto-Aurignacian occupation, EJF to an Early Aurignacian occupation, and EJM and EJJ to two distinct Evolved Aurignacian occupations. EJOP inf and EJO inf are small occupations for which the cultural attribution is less secure.
Fig. 3.
The Saint-Césaire stratigraphy (modified from ref. 24, pp. 10 and 11). From bottom to top: EGPF corresponds to a Denticulate Mousterian occupation, EJOP sup to a Châtelperronian occupation, EJO sup to a Proto-Aurignacian occupation, EJF to an Early Aurignacian occupation, and EJM and EJJ to two Evolved Aurignacian occupations. EJOP inf and EJO inf are small occupations with unclear cultural attribution.
The abundance of burned and cut-marked specimens and the low incidence of carnivore remains and carnivore-modified bones in the faunal samples indicate that humans were the main accumulators of the faunal remains at Saint-Césaire (25). Furthermore, the high δ15N value found in western European Neanderthals, including the St Césaire 1 specimen (11), indicate that most of their dietary proteins came from large herbivores. This finding strengthens the use of the Saint-Césaire fauna for examining changes in human population densities in Late Pleistocene Europe. Lastly, a study of bone refits has shown that occupation mixing has been limited at Saint-Césaire (26). These results support the potential of the stratigraphy of this site for addressing fine-grain research questions.
Reindeer, steppe bison, and horse represent 96% of total species composition for the Saint-Césaire occupations (Table 2). These three species are well represented in the Denticulate Mousterian (EGPF), Châtelperronian? (EJOP inf), and Châtelperronian (EJOP sup) occupations. In contrast, reindeer increased dramatically in abundance in the overlying Aurignacian occupations, reaching percentages from 69% to 85%. This increase is highly significant (Châtelperronian versus Proto-Aurignacian; ts = 23.27, P < 0.0001).
Table 2.
Large species and micromammal species composition in the Saint-Césaire levels
| Mousterian EGPF | Châtelperronian? EJOP inf | Châtelperronian EJOP sup | Proto-Aurignacian EJO sup | Aurignacian I EJF | Evolved Aurignacian EJM | Evolved Aurignacian EJJ | |
|---|---|---|---|---|---|---|---|
| Large species | (n = 866) | (n = 285) | (n = 803) | (n = 411) | (n = 3,432) | (n = 829) | (n = 327) |
| Reindeer | 24.7 | 33.0 | 20.2 | 84.9 | 82.3 | 72.4 | 69.1 |
| Steppe bison | 38.0 | 35.8 | 48.7 | 4.6 | 4.8 | 9.4 | 11.9 |
| Red deer | 1.0 | 2.5 | 5.1 | 0.5 | 0.3 | 0.2 | 0.6 |
| Megaceros | 0.8 | 0.4 | — | — | 0.2 | — | — |
| Roe deer | — | — | 0.5 | — | — | — | — |
| Wild boar | — | 0.4 | 0.5 | — | 0.0 | — | — |
| Horse | 34.1 | 26.3 | 17.4 | 5.4 | 11.2 | 13.6 | 17.4 |
| Wooly rhino | 0.2 | 0.4 | 3.5 | 0.7 | 0.2 | 0.1 | — |
| Wild ass | — | — | 0.2 | — | — | — | — |
| Mammoth | 0.8 | 0.7 | 2.6 | 1.9 | 0.5 | 4.0 | 0.3 |
| Spotted hyena | 0.2 | — | 0.4 | — | 0.0 | — | 0.3 |
| Wolf | 0.1 | 0.7 | 0.2 | 0.7 | 0.3 | 0.1 | 0.3 |
| Arctic fox | — | — | 0.2 | 0.2 | 0.1 | — | — |
| Polecat | — | — | 0.1 | 0.2 | 0.0 | — | — |
| Pine marten | — | — | — | 0.5 | — | — | — |
| Lynx | — | — | — | — | 0.0 | — | — |
| Badger | — | — | — | — | 0.0 | 0.1 | — |
| Cave lion | — | — | 0.2 | — | 0.1 | — | — |
| Hare | — | — | — | 0.2 | — | — | — |
| Total | 99.9 | 100.2 | 99.8 | 99.8 | 100.0 | 99.9 | 99.9 |
| Micromammals | (n = 9) | (n = 2) | (n = 38) | (n = 84) | (n = 69) | (n = 100) | (n = 109) |
| Narrow-skulled vole | 55.6 | 50.0 | 76.3 | 95.2 | 85.5 | 93.0 | 89.0 |
| Common vole | 22.2 | — | 10.5 | 1.2 | 1.4 | — | 6.4 |
| Ground squirrel | — | — | — | — | 1.4 | 1.0 | 0.9 |
| Snow vole | 11.1 | — | — | — | — | — | — |
| Water vole | 11.1 | 50.0 | 13.2 | 3.6 | 11.6 | 4.0 | 0.9 |
| Pine vole | — | — | — | — | — | . | 0.9 |
| Garden dormouse | — | — | — | — | — | 1.0 | . |
| Root/Male vole | — | — | — | — | — | 1.0 | 0.9 |
| Collared lemming | — | — | — | — | — | — | 0.9 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 99.9 | 100.0 | 99.9 |
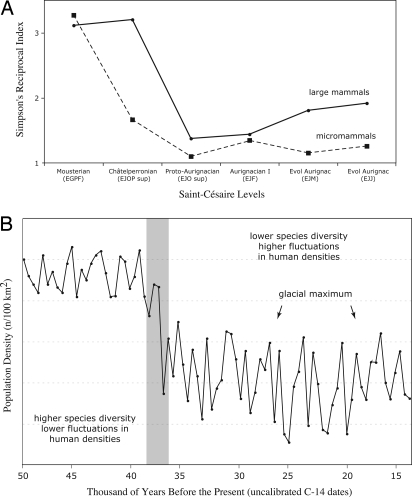
The temporal increase in reindeer abundance at Saint-Césaire is associated with a sharp decline in species diversity (Fig. 4a). This contraction of the species spectrum is consistent with the hypothesis of a climatic deterioration during the M/UP transition (10) because it is not correlated with sample size (rs = −0.03, P > 0.05). However, cultural factors—for instance, specialization on reindeer (27)—also may explain these temporal patterns. To examine this possibility, micromammal species (<500 g) recovered from the Saint-Césaire occupations were used as control data and compared with the other larger species at the site. Micromammals are helpful because they typically represent background deposition in Pleistocene assemblages and are sensitive to subtle climatic changes (28).
Fig. 4.
Faunal diversity at Saint-Césaire and its presumed impact on human densities. (A) Large mammal versus micromammal species diversity, as measured by the reciprocal of Simpson's index, in the Saint-Césaire levels. Data are from Table 2 (EJOP inf and EJO inf assemblages excluded because of small sample size). (B) Hypothetical reconstruction of fluctuations of hunter–gatherer densities in western Europe during the Late Pleistocene. The reconstruction is based on an extrapolation of the presumed effects of mammal diversity on human population densities. The shaded area shows the transition between the Châtelperronian and the Early Aurignacian.
The narrow-skulled vole (Microtus gregalis), common vole (Microtus arvalis), and water vole (Arvicola terrestris) comprise 98% of the micromammal species identified at Saint-Césaire (Table 2). Other micromammal taxa (n = 6) are poorly represented. Today, the narrow-skulled vole is restricted to Palearctic tundra and wooded steppe habitats, whereas the common vole and water vole are typical of more temperate environments (29). If a climatic deterioration induced a decline in large species diversity during the EUP, the same factor is expected to have reduced micromammal species diversity as well, based on latitudinal trends in modern analogues (8). Furthermore, if the climatic hypothesis is correct, the trend toward increasing reindeer abundance at Saint-Césaire should be associated with a significant increase in the abundance of cold-adapted micromammals.
Both predictions are met by the data. The increasing abundance of reindeer in the Saint-Césaire sequence is matched by a concomitant increase in the abundance of the narrow-skulled vole, a cold-adapted species. Importantly, the decline in the diversity of large species in the EUP of Saint-Césaire is accompanied by a marked decline in micromammal species diversity (Fig. 4a). The correlation between micromammal species diversity and sample size is not statistically significant (rs = −0.77, P > 0.05). These data indicate that changes in faunal composition at Saint-Césaire were induced by increasingly cool climatic conditions. These faunal changes are in agreement with those observed at Roc de Combe and Grotte XVI (10, 12) in France, and they suggest that this climatic deterioration occurred on a regional scale. Additionally, the high number of reindeer-dominated Aurignacian assemblages in the same region (27) lends support to the climatic deterioration inference.
Discussion
Assuming that the ecological relationship found between hunter–gatherer density and mammalian species diversity extends to the past, the reduction of species diversity in the EUP of Saint-Césaire is likely to have resulted in significant demographic changes in human populations. Human population densities not only likely declined during this period, but they also were subject to more extreme fluctuations relative to the final Mousterian (Fig. 4b). These high-amplitude fluctuations would have been induced by a growing reliance on Rangifer, a highly fluctuating resource. This evidence suggests that the EUP in the region north of the Pyrenees and the Alps corresponded to a period of niche contraction for human populations. On a broader temporal scale, faunal (10, 30) and climatic (31, 32) data indicate that this EUP pattern continued through much of the western European Upper Paleolithic, because little evidence for an expansion of the diet breadth exists for this region before ≈16,000 BP (33, 34). Specifically, fluctuations in the number of humans are expected to have been most extreme, and densities lowest, during the last Glacial Maximum (27,000–16,000 BP), when northern Europe became depopulated (35, 36).
These data also have implications for the human biological transition at the time of the M/UP archeological transition. The contribution of archaic sapiens, especially the Neanderthals, to the modern human phylogeny is the subject of considerable debate. The “replacement hypothesis” has stipulated that archaic sapiens were replaced 40,000 to 35,000 years ago by modern humans expanding out of Africa (see references in ref. 37). However, recent studies suggest that the level of gene flow between these populations might have been underestimated (38–41). The results presented here are difficult to reconcile with the “replacement” (demic expansion) hypothesis.
In France, the purported modern human expansion is said to have occurred during the Châtelperronian and Early Aurignacian (27). However, the Saint-Césaire data suggest that a climatic deterioration during this period fueled a contraction of the human niche. In this context, the probability that a large-scale expansion into an occupied territory occurred and met with success appears to be low. Futhermore, given that the M/UP transition is marked by great stability in subsistence strategies (25, 30). Therefore, although replacement of local populations by out-of-Africa migrants might have contributed in shaping human biological evolution in western Europe during the EUP, this factor was probably of secondary importance.
A scenario emphasizing bottlenecks, genetic drift, and gene flow therefore is favored over population replacement hypotheses for explaining the M/UP transition. It is hypothesized that the contraction of the human niche during the EUP promoted recurrent bottlenecks in western Europe. Combined with genetic drift, pervasive in small populations (1), these recurrent bottlenecks could account for the progressive loss of archaic traits in the local populations. The small and decreasing effective size of western European human populations versus the much larger, and possibly expanding, effective size of the human populations from Africa may have contributed to the process (42). Furthermore, the inferred spatial extension of social relations in the Aurignacian (27), a strategy that helps to buffer resource stress (43), might have contributed to the rise in frequency of modern human traits in the declining populations.
Conclusion
Terrestrial ecosystems changed greatly during the EUP in western Europe. The Saint-Césaire data suggest that a climatically induced reduction of species diversity in the EUP caused declines in human population densities. Furthermore, a reliance on reindeer, a highly fluctuating resource, would have promoted recurrent bottlenecks in the thinly scattered populations spread north of the Pyrenees and the Alps. These data also suggest that models building on in situ mechanisms, particularly population bottlenecks and genetic drift, may provide more satisfactory explanations of the evolutionary pathways taken by western European human populations during the M/UP transition than those relying mostly on demic expansion or replacement scenarios.
Materials and Methods
Ungulate abundance is estimated based on the number of identified specimens, whereas the minimum number of individuals is used to measure rodent abundance. Percentage comparisons of ungulate abundance are made by using the arcsine transformation [denoted Ts (ref. 44, pp. 419–422)]. Species diversity is evaluated based on the reciprocal of Simpson's index (1/D). D is calculated as follows:
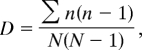 |
where n is the total number of identified remains for a particular species and N the total number of identified remains for all species. Unspecified taxa and fish species were excluded.
Because Middle Paleolithic and EUP foragers rarely included animal resources other than mammals in their diet, nonmammalian species were excluded from the analysis of the ethnographic and wildlife data. In these comparisons, terrestrial mammalian species include all native species present today at the specified coordinates, marine mammals excluded. Human groups for which hunting represented <45% of their subsistence activities, following the classification of Murdoch (45), also were excluded. A higher hunting threshold (e.g., 80%) was not adopted here to preserve adequate sample size.
Some of the human density estimates used in the analysis here are very rough, making these data less than optimal. For instance, many estimates are derived from 18th and 19th century accounts made by travelers and missionaries. Moreover, some of these estimates were produced in the postcontact period, and therefore are probably underestimations because of the negative effects of diseases and the geographical expansion of Europeans on native populations [see Keeley (4) for a fuller discussion of these and other limitations concerning these estimates]. Also, historical shifts in wild mammal distribution induced by human settlement and overhunting (e.g., the dramatic range reduction of bison in the 19th century) and other factors (e.g., the introduction of firearms and horses) also affect the accuracy of the correlations. Despite these important limitations, it is thought that they do not completely alter the overall structure of the data.
ACKNOWLEDGMENTS.
I thank Serge Couturier, Cédric Beauval, Frank Miller, John Speth, and Robert Whallon for comments on earlier versions of this manuscript and François Lévêque and Jean-Claude Marquet for discussions on Saint-Césaire. Funding for the analysis of the Saint-Césaire material was provided by the Fonds Québécois de la Recherche sur la Société et la Culture, the Social Science and Humanities Research Council of Canada, the National Science Foundation, the Service Régional d'Archéologie, Poitou-Charentes region, and the CELAT Research Center, Université Laval.
Footnotes
The authors declare no conflict of interest.
References
- 1.Futuyma DJ. Evolutionary Biology. 3rd Ed. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 2.Stiner MC, Munro ND, Surovell TA, Tchernov E, Bar-Yosef O. Science. 1999;283:190–194. doi: 10.1126/science.283.5399.190. [DOI] [PubMed] [Google Scholar]
- 3.Relethford JH. Genetics and the Search for Modern Human Origins. New York: Wiley; 2001. [Google Scholar]
- 4.Keeley LH. J Anth Arch. 1988;7:373–411. [Google Scholar]
- 5.Burch E. Am Antiq. 1972;37:339–368. [Google Scholar]
- 6.Kelly RL. The Foraging Spectrum. Washington, DC: Smithsonian Inst Press; 1995. [Google Scholar]
- 7.Huston M. Biological Diversity: The Coexistence of Species on Changing Landscapes. Cambridge, UK: Cambridge Univ Press; 1994. [Google Scholar]
- 8.Badgley C, Fox DL. J Biogeogr. 2000;27:1437–1467. [Google Scholar]
- 9.Whittaker RJ, Nogués-Bravo D, Araújo MB. Glob Ecol Biogeogr. 2007;16:76–89. [Google Scholar]
- 10.Grayson DK, Delpech F, Rigaud JP, Simek JF. J Arch Sci. 2001;28:115–125. [Google Scholar]
- 11.Bocherens H, Drucker DG, Billiou D, Patou-Mathis M, Vandermeersch B. J Hum Evol. 2005;49:71–87. doi: 10.1016/j.jhevol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Delpech F. Les Faunes du Paléolithique Supérieur Dans le Sud-Ouest de la France. Bordeaux, France: Centre National de la Recherche Scientifique; 1983. [Google Scholar]
- 13.Gunn A. Rangifer. 2003;14:105–111. [Google Scholar]
- 14.Boudreau S, Payette S, Morneau C, Couturier S. Arct Antarc Alp Res. 2003;35:187–195. [Google Scholar]
- 15.Couturier S, Jean D, Otto R, Rivard S. Demography of the Migratory Tundra Caribou (Rangifer tarandus) of the Nord-du-Québec Region and Labrador. de la Faune et des Parcs, Québec: Ministère des Ressources Naturelles; 2004. [Google Scholar]
- 16.Valkenburg P, Sellers RA, Squibb RC, Woolington JD, Aderman AR, Dale BW. Rangifer. 2003;14:131–142. [Google Scholar]
- 17.Meldgaard M. Medd Grønl Biosc. 1986;20:1–88. [Google Scholar]
- 18.Miller FL, Gunn A. Arctic. 2003;56:381–390. [Google Scholar]
- 19.Aanes R, Sæther BE, Solberg EJ, Aanes S, Strand O, Øritsland NA. Can J Zool. 2003;81:103–110. [Google Scholar]
- 20.Post E. Ecology. 2005;86:2320–2328. [Google Scholar]
- 21.Syroechkovskii EE. Wild Reindeer. Washington, DC: Smithsonian Inst Press; 1995. [Google Scholar]
- 22.Lévêque F, Vandermeersch B. C R Acad Sci Paris. 1980;291:187–189. [Google Scholar]
- 23.Mercier N, Valladas H, Joron JL, Reyss JL, Lévêque F, Vandermeersch B. Nature. 1991;351:737–739. doi: 10.1038/351737a0. [DOI] [PubMed] [Google Scholar]
- 24.Lévêque F, Backer AM, Guilbaud M. Context of a Late Neandertal: Implications of Multidisciplinary Research for the Transition to Upper Paleolithic Adaptations at Saint-Césaire, Charente-Maritime, France. Madison, WI: Prehistory Press; 1993. [Google Scholar]
- 25.Morin E. Ann Arbor: Univ of Michigan; 2004. PhD thesis. [Google Scholar]
- 26.Morin E, Tsanova T, Sirakov N, Rendu W, Mallye JB, Lévêque F. J Arch Sci. 2005;32:1083–1098. [Google Scholar]
- 27.Mellars P. The Neanderthal Legacy: An Archaeological Perspective From Western Europe. Princeton: Princeton Univ Press; 1996. [Google Scholar]
- 28.Marquet JC. Paléoenvironnement et Chronologie des Sites du Domaine Atlantique Français d'Âge Pléistocène Moyen et Supérieur d'Après l'étude des Rongeurs. Tours, France: Les Cahiers de la Claise; 1993. [Google Scholar]
- 29.Wilson DE, Reeder DM. Mammal Species of the World. 3rd Ed. Baltimore: John Hopkins Univ Press; 2005. [Google Scholar]
- 30.Grayson DK, Delpech F. J Arch Sci. 2003;30:1633–1648. [Google Scholar]
- 31.Barron E, van Andel TH, Pollard D. In: Neanderthals and Modern Humans in the European Landscape During the Last Glaciation. van Andel TH, Davies WD, editors. Cambridge, UK: McDonald Inst for Arch Res; 2003. pp. 57–78. [Google Scholar]
- 32.Guiot J, Cheddadi R. C R Geosci. 2004;336:667–675. [Google Scholar]
- 33.Laroulandie V. Talence, France: Université de Bordeaux I; 2000. PhD thesis. [Google Scholar]
- 34.Cochard D. Talence, France: Université de Bordeaux I; 2004. PhD thesis. [Google Scholar]
- 35.Jochim M. In: The Pleistocene Old World: Regional Perspectives. Soffer O, editor. New York: Plenum; 1987. pp. 317–331. [Google Scholar]
- 36.Conard NJ, Bolus M. J Hum Evol. 2003;44:331–371. doi: 10.1016/s0047-2484(02)00202-6. [DOI] [PubMed] [Google Scholar]
- 37.Trinkaus E. Ann Rev Anth. 2005;34:207–230. [Google Scholar]
- 38.Garrigan D, Mobasher Z, Severson T, Wilder JA, Hammer MF. Mol Biol Evol. 2005;22:189–192. doi: 10.1093/molbev/msi013. [DOI] [PubMed] [Google Scholar]
- 39.Hardy J, Pittman A, Myers A, Gwinn-Hardy K, Fung HC, De Silva R, Hutton M, Duckworth J. Biochem Soc Trans. 2005;33:582–585. doi: 10.1042/BST0330582. [DOI] [PubMed] [Google Scholar]
- 40.Trinkaus E. Proc Natl Acad Sci USA. 2007;104:7367–7372. doi: 10.1073/pnas.0702214104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zilhão J. Evol Ant. 2006;15:183–195. [Google Scholar]
- 42.Hawks J, Hunley K, Lee SH, Wolpoff MH. Mol Bio Evol. 2000;17:2–22. doi: 10.1093/oxfordjournals.molbev.a026233. [DOI] [PubMed] [Google Scholar]
- 43.Whallon R. J Anth Arch. 2006;25:259–270. [Google Scholar]
- 44.Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd Ed. New York: Freeman; 1995. [Google Scholar]
- 45.Murdoch GP. Atlas of World Cultures. Pittsburgh: Univ Pittsburgh Press; 1981. [Google Scholar]
- 46.Sturtevant W. Handbook of North American Indians. Washington, DC: Smithsonian Inst Press; 1978–2004. [Google Scholar]
- 47.Bergerud AT, Jakimchuk RD, Carruthers DR. Arctic. 1984;37:7–22. [Google Scholar]
- 48.Valkenburg P, Keech MA, Sellers RA, Tobey RW, Dale BW. Investigation of Regulating and Limiting Factors in the Delta Caribou Herd. Juneau: Alaska Dept of Fish and Game; 2002. [Google Scholar]
- 49.Messier F, Huot J, Le Hénaff D, Luttich S. Arctic. 1988;41:279–287. [Google Scholar]
- 50.Klokov K. In: Family-Based Reindeer Herding and Hunting Economies and the Status and Management of Wild Reindeer/Caribou Populations. Ulvevadet B, Klokov K, editors. Tromsø, Norway: Centre for Saami Studies, Univ of Tromsø; 2004. pp. 55–94. [Google Scholar]