Abstract
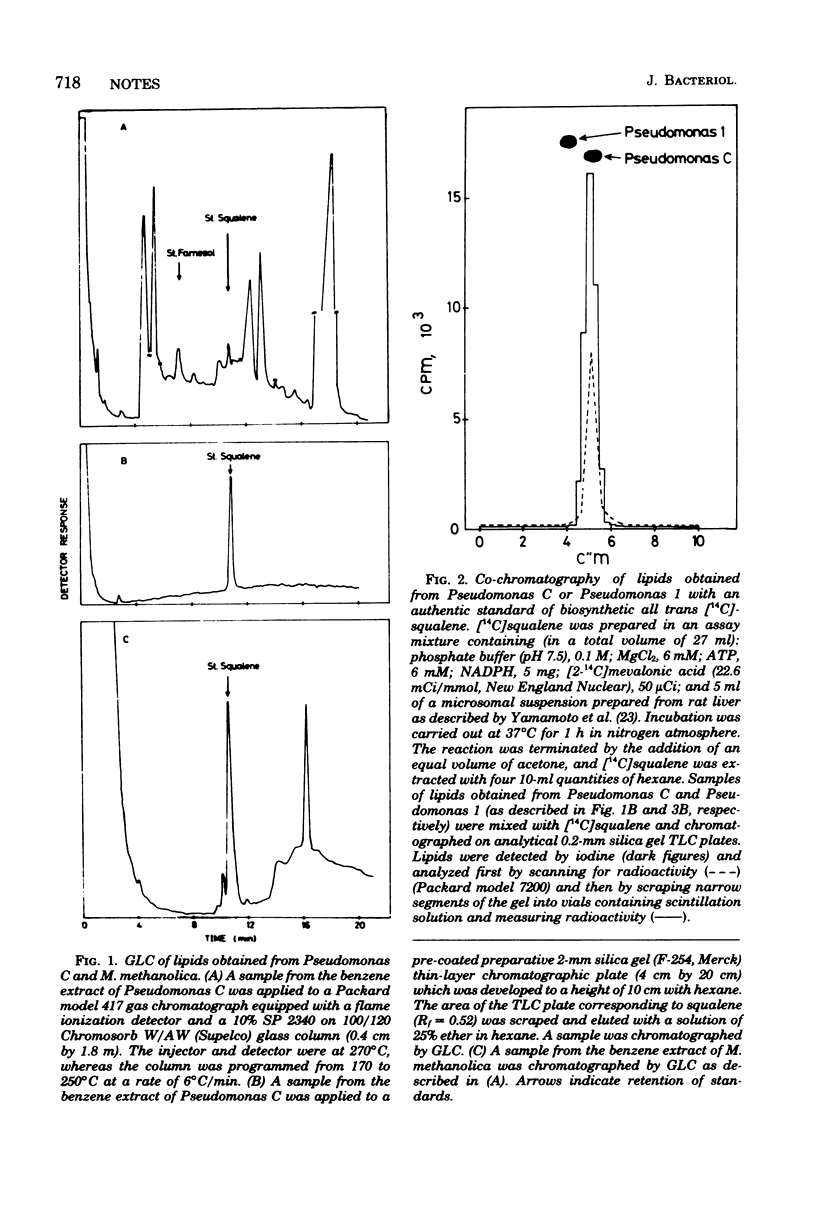
The nonpolar lipids of methanol-grown bacteria which utilize one-carbon (C1) compounds via the RMP pathway (Pseudomonas C, Pseudomonas methylotropha, and Methylomonas methanolica) were found to contain squalene in concentrations between 0.1 to 1.16 mg/g of cell (dry weight). Squalene could not be detected in lipid extracts of methanol-grown bacteria which utilize C1 compounds via the serine pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battat E., Goldberg I., Mateles R. I. Growth of Pseudomonas C on C1 compounds: continuoous culture. Appl Microbiol. 1974 Dec;28(6):906–911. doi: 10.1128/am.28.6.906-911.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird C. W., Lynch J. M., Pirt F. J., Reid W. W. Steroids and squalene in Methylococcus capsulatus grown on methane. Nature. 1971 Apr 16;230(5294):473–474. doi: 10.1038/230473a0. [DOI] [PubMed] [Google Scholar]
- Bouvier P., Rohmer M., Benveniste P., Ourisson G. Delta8(14)-steroids in the bacterium Methylococcus capsulatus. Biochem J. 1976 Nov;159(2):267–271. doi: 10.1042/bj1590267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney C. L., Levine D. W. Microbial utilization of methanol. Adv Appl Microbiol. 1972;15:337–365. doi: 10.1016/s0065-2164(08)70096-0. [DOI] [PubMed] [Google Scholar]
- De Souza N. J., Nes W. R. Sterols: isolation from a blue-green alga. Science. 1968 Oct 18;162(3851):363–363. doi: 10.1126/science.162.3851.363. [DOI] [PubMed] [Google Scholar]
- Förster H. J., Biemann K., Haigh W. G., Tattrie N. H., Colvin J. R. The structure of novel C35 pentacyclic terpenes from Acetobacter xylinum. Biochem J. 1973 Sep;135(1):133–143. doi: 10.1042/bj1350133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Jensen A. P. Phospholipid and fatty acid composition of methanol-utilizing bacteria. J Bacteriol. 1977 Apr;130(1):535–537. doi: 10.1128/jb.130.1.535-537.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Rock J. S., Ben-Bassat A., Mateles R. I. Bacterial yields on methanol, methylamine, formaldehyde, and formate. Biotechnol Bioeng. 1976 Dec;18(12):1657–1668. doi: 10.1002/bit.260181202. [DOI] [PubMed] [Google Scholar]
- Haigh W. G., Förster H. J., Biemann K., Tattrie N. H., Colvin J. R. Induction of orientation of bacterial cellulose microfibrils by a novel terpenoid from Acetobacter xylinum. Biochem J. 1973 Sep;135(1):145–149. doi: 10.1042/bj1350145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. K., Kushwaha S. C., Kates M. Structure ditermination of the squalene, dihydrosqualene and tetrahydrosqualene in Halobacterium cutirubrum. Biochim Biophys Acta. 1972 May 23;270(1):103–110. doi: 10.1016/0005-2760(72)90183-x. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Pugh E. L., Kramer J. K., Kates M. Isolation and identification of dehydrosqualene and C 40 -carotenoid pigments in Halobacterium cutirubrum. Biochim Biophys Acta. 1972 Mar 23;260(3):492–506. doi: 10.1016/0005-2760(72)90064-1. [DOI] [PubMed] [Google Scholar]
- Reitz R. C., Hamilton J. G. The isolation and identification of two sterols from two species of blue-green algae. Comp Biochem Physiol. 1968 May;25(2):401–416. doi: 10.1016/0010-406x(68)90349-6. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W., Harrison J. E., Wadzinski A. M. Metabolism of single carbon compounds. Annu Rev Microbiol. 1970;24:135–158. doi: 10.1146/annurev.mi.24.100170.001031. [DOI] [PubMed] [Google Scholar]
- Schubert K., Rose G., Hörhold C. Cholesterin in Streptomyces olivaceus. Biochim Biophys Acta. 1967 Feb 14;137(1):168–171. [PubMed] [Google Scholar]
- Schubert K., Rose G., Wachtel H., Hörhold C., Ikekawa N. Zum Vorkommen von Sterinen in Bakterien. Eur J Biochem. 1968 Jul;5(2):246–251. doi: 10.1111/j.1432-1033.1968.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Suzue G., Tsukada K., Nakai C., Tanaka S. Presence of squalene in Staphylococcus. Arch Biochem Biophys. 1968 Mar 11;123(3):644–644. doi: 10.1016/0003-9861(68)90187-2. [DOI] [PubMed] [Google Scholar]
- Suzue G., Tsukada K., Tanaka S. Occurrence of dehydrosqualene (C30 phytoene) in Staphylococcus aureus. Biochim Biophys Acta. 1968 Sep 2;164(1):88–93. doi: 10.1016/0005-2760(68)90074-x. [DOI] [PubMed] [Google Scholar]
- TCHEN T. T., BLOCH K. On the mechanism of enzymatic cyclization of squalene. J Biol Chem. 1957 Jun;226(2):931–939. [PubMed] [Google Scholar]
- Tornabene T. G., Kates M., Gelpi E., Oro J. Occurrence of squalene, di- and tetrahydrosqualenes, and vitamin MK8 in an extremely halophilic bacterium, Halobacterium cutirubrun. J Lipid Res. 1969 May;10(3):294–303. [PubMed] [Google Scholar]
- Yamamoto S., Lin K., Bloch K. Some properties of the microsomal 2,3-oxidosqualene sterol cyclase. Proc Natl Acad Sci U S A. 1969 May;63(1):110–117. doi: 10.1073/pnas.63.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]


