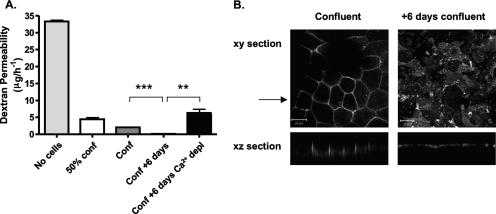
FIG. 1.
Paracellular permeability and CD26 localization in Caco-2 cells. (A) Caco-2 cells were seeded onto 0.4-μm-pore-size Transwells to achieve different levels of cell confluence, i.e., a 50% confluent monolayer (50% conf), a confluent monolayer (Conf), and a confluent monolayer maintained for an additional 6 days (Conf + 6 days). At 16 h prior to the assay, duplicate Transwells (Conf + 6 days) were incubated in calcium-free medium plus 0.5 mM EGTA to disrupt TJs. FITC-dextran (4 kDa) was added to the medium in the upper reservoir on the apical side of the cell monolayer. After 3 h, aliquots of the basolateral medium were removed and the concentration of FITC-dextran was determined. The results show the mean ± SEM of triplicate samples for each condition. **, P < 0.001; ***, P < 0.0001 (t test). (B) Caco-2 cells were cultured on 0.4-μm-pore-size Transwells until confluent, fixed with methanol or cultured for an additional 6 days, and then fixed for antibody staining. Cells were stained with anti-CD26 antibody, and bound antibody was visualized with anti-mouse Ig-Alexa 488 secondary antibody and confocal microscopy. The large panels represent x-y sections, and the small panels are x-z sections, where the arrow indicates the plane the z section was taken from. z sections were compiled by taking 0.76-μm steps through each x-y section. The scale bars represent 20 μm.