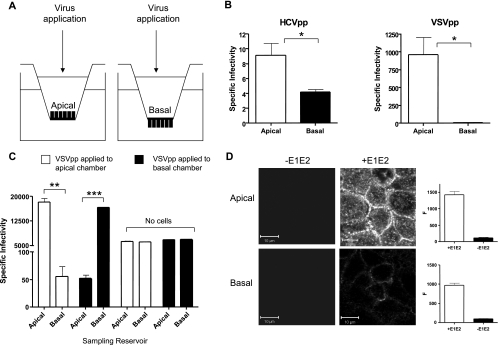
FIG. 6.
Effect of polarity on HCVpp infection. (A) Caco-2 cells were grown until polarized on 0.4-μm-pore-size Transwell inserts, allowing access to the apical or basal membrane for HCVpp or VSVpp infection. (B) HCVpp and VSVpp infection of polarized Caco-2 cells via the apical or basal surface. Data are expressed as specific infectivity where the ratio of luciferase activity for HCVpp or control VSVpp to Env− particles is shown. (C) To control for virus mixing between the apical and basal compartments, the medium was removed from both reservoirs after an 8-h VSVpp infection and infectivity was assessed by inoculating Huh-7.5 cells. As a control, VSVpp was applied to the upper and lower reservoirs of Transwells with no cells. Ninety-six percent of the VSVpp applied to the apical surface of polarized Caco-2 cells (white bars) remained in the apical reservoir. The result was similar when virus was applied to the basal reservoir (black bars). In the absence of cells, infectious VSVpp were found equally distributed between the upper and lower chambers. (D). HCV strain HCV-1 E1E2 was applied to the apical or basal surface of polarized Caco-2 cells at 3 μg/ml in PBS and incubated for 1 h at 37°C. Unbound antigen was removed by washing, and the cells were fixed with ice-cold methanol. Bound antigen was visualized with rat anti-E2 MAbs 9/75 and 7/59, anti-rat-Alexa 633, and confocal microscopy. Bound MAbs were quantified with Zeiss LSM line plot software, and the data are expressed as arbitrary fluorescence units (F). The upper and lower parts of the panel show the binding of E1E2 when applied to the apical or basal surface, respectively. The scale bars represent 10 μm. **, P < 0.001; ***, P < 0.0001.