Abstract
Many viruses regulate gene expression, both globally and specifically, to achieve maximal rates of replication. During herpes simplex virus 1 infection, translation of the DNA polymerase (Pol) catalytic subunit is inefficient relative to other proteins of the same temporal class (D. R. Yager, A. I. Marcy, and D. M. Coen., J. Virol. 64:2217-2225, 1990). To investigate the mechanisms involved in the inefficient translation of Pol and to determine whether this inefficient translation could affect viral replication, we performed a mutagenic analysis of the 5′ end of the pol transcript. We found that a short sequence (∼55 bases) in the 5′ leader of the transcript is both necessary and sufficient to inhibit translation in rabbit reticulocyte lysates and sufficient to inhibit reporter gene translation in transfected cells. RNase structure mapping experiments indicated that the inhibitory element adopts a structure that contains regions of a double-stranded nature, which may interfere with ribosomal loading and/or scanning. Pol accumulated to ∼2- to 3-fold-higher levels per mRNA in cells infected with a mutant virus containing a deletion of the ∼55-base inhibitory element than in cells infected with a control virus containing this element. Additionally, the mutant virus replicated less efficiently than the control virus. These results suggest that the inhibitory element regulates Pol translation during infection and that its inhibition of Pol translation is beneficial for viral replication.
Efficient viral replication generally depends on virally encoded activities that alter viral and cellular gene expression, both specifically and globally. Herpes simplex virus 1 (HSV-1) expresses its genes during productive infection in a tightly controlled cascade pattern (13). Although much of the regulation of gene expression during productive HSV-1 infection is at the level of transcription, some genes are regulated posttranscriptionally (10, 28). These additional levels of regulation may allow the virus to have more control over the expression of certain genes that are especially critical to the viral life cycle.
One HSV-1 gene whose expression is regulated posttranscriptionally encodes the catalytic subunit of the viral DNA polymerase (Pol) (28). It is interesting to note that several other viral polymerases are also translated inefficiently or otherwise posttranscriptionally regulated (reviewed in reference 7), suggesting that keeping polymerase levels low during infection may be a general strategy utilized by viruses and thus may be important to achieve maximal rates of viral replication. HSV-1 Pol is expressed as an early gene product. However, its accumulation and rate of synthesis relative to the accumulation of its mRNA are low compared with those of other early proteins, such as the single-stranded DNA binding protein ICP8 and the viral thymidine kinase (27, 28). Moreover, Pol synthesis decreases drastically at 6 h postinfection (hpi), at which time pol transcripts accumulate to their highest levels (29). At 6.5 hpi, only a small fraction of pol transcripts is associated with polyribosomes (28), indicating that the inefficient translation of Pol at this time is likely due to a defect in the initiation of translation.
The molecular mechanisms responsible for the inefficient translation of HSV-1 Pol are poorly understood. To investigate the inhibitory mechanism, the 5′ end of the pol transcript was mapped and cis-acting sequences with the potential to inhibit the initiation of Pol translation were identified (28). These elements include a very long untranslated segment containing a short upstream open reading frame (ORF), high G+C content, and extensive and stable predicted secondary structure. Deletion of the majority of the pol 5′ leader, but with the first 55 bases intact, has been reported to increase Pol translation from synthetic transcripts in vitro (9), suggesting the presence of an inhibitory element. However, a similar deletion in HSV-1 did not result in overexpression of Pol in infected cells (29). The latter result suggested that the first ∼55 bases of the pol 5′ leader may contain an element that inhibits Pol translation. The high G+C content (∼80%) of the first ∼55 bases led us further to hypothesize that these bases adopt a secondary structure which might act to inhibit either the loading or scanning of initiating ribosomes. This is a mechanism utilized to regulate the expression of multiple other genes at the level of translation (17). Additionally, we hypothesized that the regulation of Pol translation is important for HSV-1 replication.
In this report, we investigate the above-mentioned hypotheses by identifying an inhibitory element in the pol 5′ leader and characterize its inhibitory effect and secondary structure by performing mutagenic analyses and RNase structure mapping experiments. We also address the importance of inefficient Pol translation to viral replication by deleting the inhibitory element from HSV-1 and assaying Pol levels and viral replication.
MATERIALS AND METHODS
Plasmids.
pINGUL30, which contains the pol ORF and all but the first ∼100 bases of the pol 5′ untranslated region (UTR), has been previously described (8). pBSpol-1 was constructed by ligating the HindIII-XbaI (all restriction endonucleases from NEB) fragment from pDP1 (20), which contains the entire pol ORF and all but the first ∼55 bases of the 5′ UTR, to HindIII-XbaI-digested and calf intestinal alkaline phosphatase (CIP; NEB)-treated pBluescript II SK+ (pBS; Stratagene). To generate pBSpol-1b, pBSpol-1 was digested with HindIII, treated with the Klenow fragment of Escherichia coli DNA Pol I (Klenow; NEB), and digested with XbaI, and the pol-containing fragment was purified and ligated into KpnI-digested, Klenow-treated, XbaI-digested, and CIP-treated pBS.
LEH.VII.5W.13 (constructed and kindly provided by Louane Hann of this laboratory) was generated by performing PCR on HSV-1 strain KOS viral DNA, using the primers pol111 (5′-TCGATGACGCGAATAAACCGGG-3′, which introduces a ClaI restriction site immediately upstream of the pol transcription start site) and 270L (5′-TTCGCTATAGTACGTATGGC-3′, which lies within the pol ORF), and cloning this PCR product into the vector pCR2.1 (Invitrogen). pBSpol-2 was created by ligating a ClaI-SnaBI fragment containing the complete pol 5′ UTR from LEH.VII.5W.13 to ClaI- and SnaBI-digested and CIP-treated pBSpol-1. pBSpol-2b was generated by digesting pBSpol-2 with ClaI, treating it with Klenow, digesting it with XbaI, purifying the pol-containing fragment, and ligating this fragment to pBS digested with KpnI, treated with Klenow, digested with XbaI, and treated with CIP. pBSpol-2c was created by removing the remaining few bases between the T7 promoter in pBS and the inserted pol sequence from pBSpol-2b by using QuikChange mutagenesis (Stratagene) according to the manufacturer's instructions. pBSpol-2-ClaI was generated from pBSpol-2 by engineering a ClaI site at the 3′ end of the pol 5′ UTR, just upstream of the pol-initiating AUG codon, using QuikChange mutagenesis.
The firefly luciferase constructs used for in vitro translation were constructed in the pBS vector. pBSpol1-57-luc was generated by purifying the pBS vector-containing fragment from pBSpol-2c following digestion with BamHI, treatment with Klenow, digestion with XbaI, and treatment with CIP and ligating this fragment to the purified luciferase ORF-containing fragment from pGL3-control (Promega) following digestion with NcoI, treatment with Klenow, and digestion with XbaI. pBSpol58-208-luc was derived from pBS-StuI, which was created by introducing a StuI restriction site immediately downstream of the T7 phage promoter in pBS by using QuikChange mutagenesis. pBSpol58-208-luc was generated by purifying the pBS vector-containing fragment from pBS-StuI following digestion with StuI and XbaI and treatment with CIP and ligating this fragment to the purified luciferase ORF-containing fragment from pGL3-pol5′UTR following digestion with BamHI, treatment with Klenow, and digestion with XbaI. pBS-StuI-luc was generated by purifying the pBS vector-containing fragment from pBS-StuI following digestion with SmaI and XbaI and treatment with CIP and ligating this fragment to the purified luciferase ORF-containing fragment from pGL3-control following digestion with NcoI, treatment with Klenow, and digestion with XbaI. To create the mutant constructs for pBSpol1-45-luc, pBSpol1-34-luc, pBSpol15-59-luc, pBSpol29-59-luc, and pBSpol15-28, oligonucleotides containing the corresponding bases of the pol 5′ UTR were synthesized (Integrated DNA Technologies) such that when annealed to their complementary sequence, the resulting double-stranded DNA oligonucleotides have a blunt 5′ end and an NcoI-compatible sticky 3′ end. These oligonucleotides were phosphorylated with polynucleotide kinase (NEB), annealed to their complementary sequence, and ligated to StuI- and NcoI-digested and CIP-treated pBS-StuI-luc. The negative-control construct, pBS-luc2, was generated by digesting pGL3-control with HindIII and XbaI, purifying the luciferase ORF-containing fragment, and ligating this fragment to HindIII/XbaI-digested and CIP-treated pBS.
The firefly luciferase constructs used in transient-transfection assays were derived from pGL3-control, which drives expression of luciferase from the simian virus 40 (SV40) promoter. pGL3-MCS was created by ligating the Klenow-treated BssHII fragment containing the multiple cloning site (MCS) from pBS into pGL3-control that was digested with NcoI and HindIII and treated with Klenow and CIP. pGL3-pol5′UTR was created by ligating a Klenow-treated ClaI fragment containing the complete pol 5′ UTR from pBSpol-2-ClaI to pGL3-control that was digested with NcoI and HindIII and treated with Klenow and CIP. pGL3-pol-uORFmut was created in the same manner as pGL3-pol5′UTR, except that the ClaI fragment used in this case had the AUG codon of the uORF mutated to AAG by using QuikChange mutagenesis. pGL3-pol1-57 was generated by ligating a Klenow- and CIP-treated BamHI fragment from pGL3-pol5′UTR (this fragment contains the first 57 bases of the pol 5′ UTR but not the firefly luciferase ORF) to a Klenow-treated, NcoI- and BamHI-digested fragment from pGL3-control (this fragment contains the luciferase ORF). pGL3-pol58-208 was created by ligating a BamHI-digested, Klenow-treated, XbaI-digested fragment from pGL3-pol5′UTR (this fragment contains pol 5′ UTR bases 58 to 208 upstream of the firefly luciferase ORF) to pGL3-control that had been digested with HindIII, treated with Klenow, digested with XbaI, and treated with CIP. The Renilla luciferase expression vector pRL-SV40 (Promega) was used as a control during transient-transfection assays.
The constructs used in marker rescue to generate the recombinant viruses used in this report were derived from the plasmid pWR.3, which was generously provided by John Balliet and Priscilla Schaffer (3). The BamHI site in the vector sequence of pWR.3 was deleted by digestion with KpnI and SpeI, treatment with Klenow, and self-ligation to create pWR.3(-BamHI). Using QuikChange mutagenesis, a new BamHI site was introduced at the extreme 5′ end of the pol 5′ UTR to create p3WB2. pW1ΔB2 was then generated by digesting p3WB2 with BamHI and self-ligating, which deletes the 53 bases between the newly introduced BamHI site and the BamHI site in the pol 5′ leader. pW1ΔB2.polΔ54.x was then generated by purifying the pol sequence-containing fragment from pW1ΔB2 following digestion with SrfI and XbaI and ligated to the purified fragment containing the pol ORF from pBSpol-2 following digestion with SrfI and XbaI. pWRΔB.pol.x was generated by purifying the pol sequence-containing fragment from pWR.3 following digestion with SrfI and XbaI and ligated to the purified fragment containing the pol ORF from pBSpol-2 following digestion with SrfI and XbaI.
The sequences of all relevant parts of plasmids generated using QuikChange mutagenesis were verified by DNA sequencing.
In vitro transcription and translation.
RNA was in vitro transcribed from linearized DNA templates and capped using an mMessage mMachine kit (Ambion) according to the manufacturer's protocol. RNA was purified following the transcription reaction by using an RNeasy kit (Qiagen). The relative concentration and quality of the in vitro-transcribed RNA were assessed by agarose gel electrophoresis and visualization with ethidium bromide staining. The capped synthetic RNA was in vitro translated in rabbit reticulocyte lysates (RRL; Ambion) at 30°C for 2 hours. For autoradiograms, samples of the translation reaction, labeled with [35S]methionine, were added to sodium dodecyl sulfate (SDS) loading buffer and heated to 95°C for 5 min prior to resolution by SDS-polyacrylamide gel electrophoresis. Gels were dried and used to expose a phosphor storage screen. Data were analyzed using Quantity One software (Bio-Rad). For luciferase assays performed using in vitro-translated samples, unlabeled, rather than radiolabeled, methionine was added to the translation reaction, and luciferase activities were measured using the BrightGlo luciferase substrate (Promega) in a Wallac Victor 2 luminometer. In each assay, equivalent amounts of the different RNAs, as determined by quantifying the intensities of the corresponding bands on an ethidium bromide-stained agarose gel with data collected on a Gel Doc system (Bio-Rad) using Quantity One software (Bio-Rad), were added to the RRL.
RNase structure mapping.
RNA was in vitro transcribed from a BamHI-linearized pBSpol-2c DNA template by using a MEGAshortscript kit (Ambion) and was purified following the transcription reaction by phenol-chloroform extraction and ethanol precipitation. This purified RNA was dephosphorylated by treatment with CIP and end labeled with polynucleotide kinase by using [γ-32P]ATP (167 μCi/μl, >7,000 Ci/mmol [ICN]), using a KinaseMax kit (Ambion). Labeled RNA was resolved on a 12% denaturing polyacrylamide gel, the full-length product was visualized by autoradiography, and the corresponding gel slice was excised and crushed with a pipette tip. RNA was eluted from the crushed gel overnight in 300 μl in crush-and-soak buffer (500 nM ammonium acetate, 10 mM magnesium acetate, 1 mM EDTA, 0.1% SDS) at 4°C, followed by a second elution step with 100 μl crush-and-soak buffer for 1 hour at 37°C. Eluted RNA was phenol-chloroform extracted, ethanol precipitated, and washed with 70% ethanol, and the pellet was resuspended in RNA renaturation buffer (10 mM NaH2PO4, 50 mM NaCl, 3 mM MgCl2, 0.1 mM EDTA). RNA was denatured by heating the sample to 80°C for 5 min and renatured by slowly cooling it to 25°C at a rate of 0.33°C/second using a thermal cycler (MJ Research). RNase digestions were performed as directed by the manufacturer (biochemistry grade RNase A, T1, and V1; Ambion). Following RNase digestion, reactions were stopped by ethanol precipitation and RNA was resuspended in loading buffer II (Ambion). Samples were resolved on a 12% sequencing gel (SequaGel; National Diagnostics). The gel was dried and used to expose a Bio-Rad phosphor storage screen. Bases that were sites of cleavage by the various RNases were used as constraints in the RNA folding algorithm mfold (32) to generate a model for the secondary structure of the RNA molecule.
Cells and viruses.
Vero (American Type Culture Collection) and polB3 cells were maintained in Dulbecco's minimal essential medium supplemented with 5% newborn calf serum, 1% penicillin and streptomycin, and 1% amphotericin B. polB3 cells, which were kindly provided by Charles Hwang, are derived from Vero cells and express HSV-1 Pol under the control of the SV40 early promoter (14). HSV-1 wild-type stain KOS was used to superinfect Vero cells in transient-transfection luciferase assays. Construction of viruses polΔ54.x and wt.x is described below.
Transient-transfection luciferase assays.
Vero cells were transfected with two plasmids, one containing the firefly luciferase gene downstream of the test 5′ leader and the other, pRL-SV40 (Promega), containing the Renilla luciferase gene as a control for transfection and lysis efficiency. Transfections were performed using Effectene reagents (Qiagen) according to the manufacturer's protocol. At 18 h posttransfection, cells were infected with HSV-1 strain KOS at a multiplicity of infection (MOI) of 10. Luciferase activity was measured at 6 hpi (24 h posttransfection). Firefly luciferase activity was normalized to the level of Renilla luciferase activity in each case. Cell lysis and addition of substrate were done according to the manufacturer's protocol (dual luciferase assay kit; Promega). Luminescence was assayed for 1 second by using a Wallac Victor 2 luminometer.
Real-time RT-PCR.
Total RNA was purified from Vero cells transfected with the various luciferase constructs by using the RNeasy kit (Qiagen). Transfected plasmid DNA was digested with Turbo DNase (Ambion), and cDNA was synthesized as previously described (18), using the reverse primer Fluc-R2 for firefly luciferase cDNA synthesis (5′-GGG GCA ACT GCA ACT CCG ATA AAT-3′) and Rluc-R3 for Renilla luciferase cDNA synthesis (5′-TAT ATT TTC CCA TTT CAT CAG GTG-3′). Real-time PCR was performed on an ABI Prism 7700 sequence detection system, using SYBR green PCR master mix (Applied Biosystems) and the primers Fluc-F2 (5′-AAT TCTTTA TGC CGG TGT TGG GCG-3′) and Fluc-R2 for firefly luciferase amplification and Rluc-F3 (5′-TCG GAC CCA GGA TTC TTT TC-3′) and Rluc-R3 for Renilla luciferase amplification. The linearity of the real-time PCR assays was demonstrated using serial dilutions of RNA purified from cells transfected with pGL3-pol5′UTR and diluted with RNA purified from mock-transfected cells. The efficiency of the real-time reverse transcription-PCR (RT-PCR) assay was approximately 2.
Virus construction.
Viruses were derived from the pol-null mutant HP66 (20), which was passaged on polB3 cells to complement the pol mutation. The pol 5′ UTR deletion mutant, polΔ54.x, was generated by performing marker rescue as described previously (6), with some modifications. Briefly, HP66 infectious DNA was cotransfected into polB3 cells along with linearized pW1ΔB2.polΔ54.x plasmid DNA by using Effectene (Qiagen) according to the manufacturer's protocol. Progeny virus from this transfection was harvested and titrated on both polB3 cells (permissive for HP66 infection) and Vero cells (restrictive for HP66 infection). The desired recombinants had the pol ORF restored and thus were able to form plaques on Vero cells. Vero cell plaques were harvested and screened for the deletion by PCR. Recombinants containing the deletion were plaque purified three times prior to performance of experiments. The wild-type control virus, wt.x, was generated in the same way, except that HP66 was cotransfected with pWRΔB.pol.x, which contains the wild-type pol sequence. The expected sequences of the pol 5′ UTR and ORF were confirmed by DNA sequencing for both the wt.x and polΔ54.x viruses.
Western blotting.
Vero cell monolayers in 60-mm cell culture dishes were either mock infected or infected at an MOI of 10 with polΔ54.x or wt.x for the times indicated. Cell lysates were prepared by washing the monolayers with phosphate-buffered saline and then scraping the Vero cells in 350 μl of SDS-polyacrylamide gel electrophoresis loading buffer. Lysates were denatured by heating them to 95°C for 5 min and resolved by polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene difluoride membrane by using a semidry Trans-Blot SD apparatus (Bio-Rad). Membranes were blocked in 5% milk and reacted with either PP5 anti-Pol (29) or 3-83 anti-ICP8 (15) antibodies followed by horseradish peroxidase-conjugated secondary antibodies (Southern Biotech). Blots were developed using enhanced chemiluminescence (Pierce). Western blotting data were quantified by scanning films and comparing band intensities to the intensities of bands in a standard curve generated from a dilution series of infected cell extracts, using Quantity One software (Bio-Rad). Pol values were normalized to ICP8 levels and were adjusted for mRNA levels, which were determined by Northern blot analyses (described below).
Northern blotting.
Total RNA was purified from mock-infected or infected cells at the time indicated, using an RNeasy kit (Qiagen). RNA samples were resolved by agarose gel electrophoresis and transferred to a positively charged nylon membrane (Hybond-N+; AP Biotech), using NorthernMax transfer buffer (Ambion), by downward capillary action. RNA was then UV cross-linked to the membrane by using a Stratalinker (Stratagene). Membranes were blocked by prehybridization with UltraHyb buffer (Ambion) at 42°C. The membrane was then incubated with a 32P-radiolabeled probe generated from a HindIII/XbaI restriction fragment from pINGUL30 (for pol) or EcoRI-linearized pSP8pA (for ICP8, generously provided by David Knipe), using a random primed DNA synthesis kit (Roche) overnight at 42°C. Membranes were washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% SDS at 42°C and then used to expose a phosphor storage screen. Quantity One software (Bio-Rad) was used to analyze the data.
Viral replication assays.
Vero cells in cell culture tubes were infected at an MOI of 0.01 with each virus assayed. Back titrations of the inocula confirmed the calculated MOIs. Viruses were harvested by freezing the tubes, followed by thawing and sonication. Cell debris was pelleted by centrifugation at low speed (∼1,000 rpm), and the supernatants were titrated on Vero cells in duplicate.
RESULTS
The first ∼55 bases of the pol 5′ leader are necessary for inhibition of Pol translation in vitro.
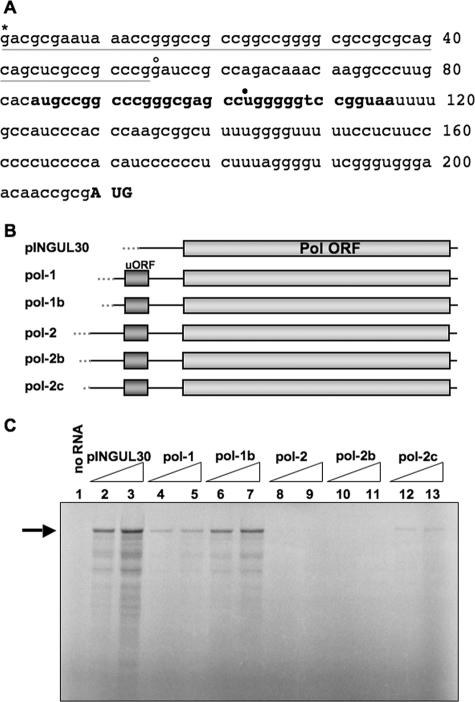
Our laboratory previously mapped the 5′ end of the pol transcript (sequence in Fig. 1A) and identified several elements that could potentially contribute to the observed inefficient initiation of Pol translation (28). Among these elements are a short upstream ORF (uORF), a long 5′ leader (>200 bases in length) that is a highly G+C-rich, extensive, and stable predicted secondary structure in this leader sequence, and a suboptimal Kozak consensus sequence surrounding the initiating AUG codon of the pol ORF (28).
FIG. 1.
Identification of an inhibitory element in the pol 5′ leader. (A) Primary nucleotide sequence of the pol 5′ UTR. The short upstream ORF is indicated in bold, and the pol-initiating AUG codon is indicated in bold capital letters. The pINGUL30 construct begins with the base indicated with a closed circle, the pol-1 constructs begin with the base indicated with the open circle, and the pol-2 constructs begin with the base indicated with the asterisk. The base indicated with the asterisk is also the base previously determined to be the start of the pol mRNA by S1 nuclease digestion and primer extension analyses (28). The bases deleted from the polΔ54.x mutant are underlined in gray. Base numbering is indicated to the right of the sequence. (B) Schematic diagram of synthetic transcripts used in in vitro translation reactions highlighting the differences in the 5′ ends of these constructs. The pol ORF is shown as a light gray box, and the pol 5′ leader is shown as a black line upstream of the ORF, with the short upstream ORF (uORF) highlighted by a dark gray box. Vector-derived bases present in the transcripts are indicated by gray dots upstream from the pol 5′ leader. pINGUL30 contains 107 pol 5′ UTR-derived bases and 68 bases derived from the transcription vector upstream of the pol AUG codon. The pol-1 constructs contain 156 pol 5′ UTR-derived bases. Pol-1 also contains 52 vector-derived bases, and pol-1b contains 11. The pol-2 constructs contain the full-length pol 5′ UTR (209 bases). Pol-2 also contains 46 vector-derived bases, pol-2b contains 11, and pol-2c contains only 1. (C) In vitro translation of the synthetic in vitro-transcribed RNAs diagrammed in panel B in RRL. Samples in lanes 2, 4, 6, 8, 10, and 12 were from reactions programmed with the same amount of RNA, and samples in lanes 3, 5, 7, 9, 11, and 13 were programmed with approximately threefold more RNA. The sample in lane 1 was from a reaction mixture incubated without an RNA template. Samples were labeled with [35S]methionine. Full-length Pol products are indicated by the arrow to the left of the gel.
To investigate whether any of these elements contribute to the regulation of Pol translation, we generated several constructs with truncations in the pol 5′ leader (Fig. 1B), which were used as templates to produce in vitro-transcribed RNA that was subsequently translated in vitro in RRL. Transcripts containing the complete pol 5′ leader (pol-2c) translated poorly in RRL (Fig. 1C, lanes 12 and 13). In contrast, transcripts containing 5′ truncations of the 5′ leader sequence (pINGUL30 and pol-1b) were translated more efficiently in RRL (Fig. 1C, lanes 2, 3, 6, and 7). The largest improvement in the efficiency of Pol translation (∼10-fold) was observed when the first ∼55 bases of the pol 5′ leader, which are approximately 80% G+C, were removed in pol-1b (Fig. 1C, compare lane 6 to lane 12 and 7 to 13). When an additional ∼50 bases of pol sequence were removed (pINGUL30), which destroys the uORF, there was an improvement in translation in RRL (Fig. 1C, compare lanes 2 and 3 to lanes 6 and 7), consistent with a previous report (9), but this improvement was slight compared with the improvement due to deletion of the first 55 bases. We observed less-than-full-length products of translation in all lanes with detectable signals (Fig. 1C, lanes 2 to 7, 12, and 13), which we attributed to premature termination of translation or degradation of the full-length product.
These data indicate that the first ∼55 bases of the pol 5′ leader are necessary to inhibit translation in vitro and suggest that these highly G+C-rich bases are responsible for the majority of the observed inhibitory activity. These data are consistent with previously published data from our laboratory that showed that deletion of bases 57 to 200 in the pol 5′ leader did not alleviate the inefficient translation of Pol in infected cells (29).
The constructs that we used to assay Pol translation in RRL contained various lengths of vector sequences derived from the MCS of pBluescript upstream from the pol 5′ UTR sequence but downstream from the promoter used to generate the corresponding RNA molecules in vitro, resulting in vector sequences at the 5′ ends of transcripts. We observed that the longer the vector-derived bases at the 5′ end of a given transcript, the less efficient the translation of that transcript in RRL (Fig. 1C, lanes 4 to 13, compare pol-1 to pol-1b and pol-2 to pol-2b and pol-2c). This observation may simply be due to an increase in the length of the 5′ UTR or may be due to the palindromic nature of the sequence present in the MCS creating a sequence or structure that acts to inhibit translation in RRL. Regardless, pol-2c, which contains only one vector-derived base, was translated inefficiently.
The first ∼55 bases of the pol 5′ leader are sufficient to inhibit translation in vitro.
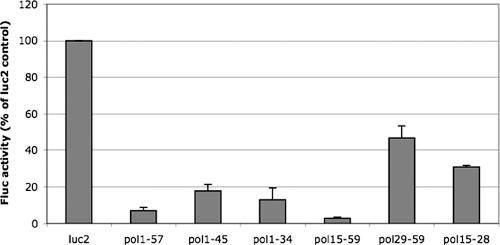
To assess whether the first ∼55 bases of the pol 5′ leader are sufficient to inhibit translation, we generated several constructs that contain portions of the pol 5′ leader upstream of the firefly luciferase ORF (refer to Fig. 1A for sequences). We assayed the effects of these various 5′ leaders by translating an equal amount of each transcript in RRL and then measuring luciferase activity. Bases 1 to 57 of the pol 5′ UTR (pol1-57) were sufficient to inhibit translation when placed upstream of the firefly luciferase ORF (Fig. 2). This inhibition was approximately 10-fold that for the negative-control 5′ UTR (Fig. 2, luc2), which contains 86 bases of vector-derived sequence as its 5′ UTR. To investigate which regions of the first ∼55 bases of the pol 5′ UTR were responsible for the inhibitory activity, we performed deletion mutagenesis on this sequence. Deleting 3′ portions of the inhibitory element (pol1-34 and pol1-45) had a modest effect on disruption of the inhibitory activity (Fig. 2). Deleting the first 14 bases (pol15-59) resulted in an increase in the inhibition of translation, while deletion of the first 28 bases (pol29-59) resulted in a substantial disruption of the inhibition (Fig. 2). Because complete inhibition was seen with the pol15-59 construct but not with the pol29-59 construct, we tested pol15-28 to determine whether bases 15 to 28 of the pol 5′ leader were responsible for this difference. Translation of the pol15-28 construct was similar to that of pol29-59 (Fig. 2), suggesting that bases 15 to 28 are important for the inhibitory activity of the pol 5′ leader but alone are not sufficient to confer full inhibition. These data indicate that bases 15 to 59 of the pol 5′ leader are sufficient to inhibit translation in vitro.
FIG. 2.
Mapping a region of the inhibitory element sufficient to confer inhibition. Synthetic transcripts encoding firefly luciferase with the listed bases from the pol 5′ leader (refer to Fig. 1A for sequences) were translated in vitro in RRL. All reactions were programmed with equivalent amounts of in vitro-transcribed RNA. Following in vitro translation, samples were assayed for luciferase activity. Luc2 RNA contains a negative-control 5′ leader 87 bases in length. Results are presented as percentages of firefly luciferase activity relative to the luc2 control levels from three independent experiments, except the results for pol15-28, which were from two experiments, with error bars representing the standard errors of the means.
Secondary structure of the inhibitory element.
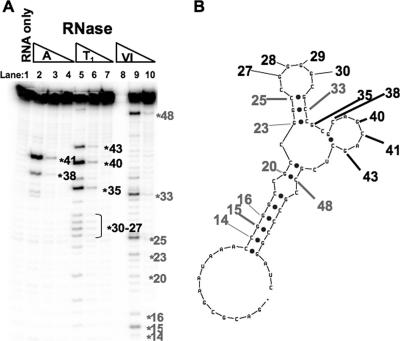
Given our hypothesis that the first ∼55 bases of the pol 5′ leader inhibit the initiation of Pol translation by forming a secondary structure, which might interfere with ribosomal loading and/or scanning, we wished to experimentally determine the secondary structure, if any, of this inhibitory RNA element. Toward this end, we generated in vitro-synthesized transcripts corresponding to the inhibitory element (pol 5′ UTR bases 1 to 58), labeled their 5′ ends, and assayed the locations in the transcripts that were sensitive and resistant to cleavage by the single-stranded specific RNases A and T1 and by the double-stranded specific RNase V1. The RNase digestion pattern indicated that the inhibitory RNA element has regions of both single-stranded and double-stranded natures (Fig. 3A). Bases 27 to 30, 35, 38, 40, 41, and 43 were sensitive to digestion by the single-strand specific RNases A and T1, and bases 14 to 16, 20, 23, 25, 33, and 48 were cleaved by the double-stranded specific RNase V1. These bases were inputted as constraints into the RNA folding algorithm mfold, and a model for the secondary structure of the inhibitory element was generated (Fig. 3B).
FIG. 3.
RNase structure mapping of the inhibitory RNA element. (A) End-labeled inhibitory element RNA (pol 5′ UTR bases 1 to 58) was partially digested with RNases A, T1, and V1, and the digestion products were resolved on a denaturing polyacrylamide gel to determine the regions of the RNA that had single-stranded or double-stranded natures. The RNA was also run untreated (lane 1). Single-stranded bases, which were sensitive to digestion by RNase A (lanes 2 and 3) or T1 (lanes 5 and 6), are numbered in black. Double-stranded bases, which were sensitive to digestion by RNase V1 (lane 9), are numbered in gray. (B) Model of the secondary structure of the inhibitory element RNA generated by the RNA folding algorithm mfold, with constraints based on data from panel A. The numbered bases from panel A are also indicated.
One notable feature of our model is that the first 12 bases of the RNA are not structured (Fig. 3B). This finding was consistent with a role for structure in the inhibitory mechanisms because these unstructured bases could be deleted without disrupting the inhibitory activity, and their deletion actually increased the inhibition (Fig. 2). Another interesting feature of the model was that the most stable region of double-stranded RNA was the one closest to the 5′ end of the molecule, bases 13 to 18 paired to bases 50 to 55 (Fig. 3B), making it the leading candidate for involvement in the inhibitory mechanism. There were also other regions predicted to form shorter stretches of double-stranded RNA. Bases 20 and 21 are predicted to be paired to bases 47 and 48, and bases 23 to 25 are predicted to be paired to bases 32 to 34 (Fig. 3B). The majority of the remaining bases were predicted to be single stranded in nature (Fig. 3B).
Activity of the inhibitory element in transfected cells in the context of HSV-1 infection.
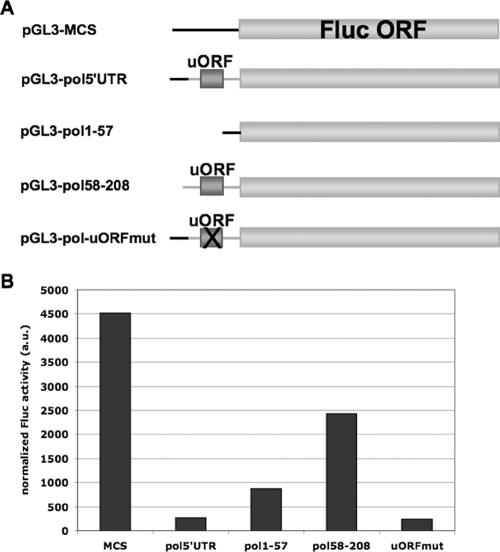
To determine whether the pol 5′ leader inhibitory element was active in transfected cells in the context of HSV-1 infection, we generated several constructs that contained pol 5′ leader sequences upstream of the firefly luciferase ORF (Fig. 4A), whose expression was driven by the SV40 promoter. Firefly luciferase activity was assayed and normalized to Renilla luciferase activity (to control for transfection efficiency) in transfected Vero cells that were subsequently infected with HSV-1 strain KOS at an MOI of 10 (Fig. 4B). Firefly luciferase transcript levels were also assessed in parallel by real time RT-PCR (see Supplemental Fig. 1 and 2 and Supplemental Table 1 at http://coen.med.harvard.edu/). When normalized to the levels of Renilla luciferase transcripts, these results showed that transcripts from most of the constructs accumulated to similar levels, although even following normalization there appeared to be reduced levels of Pol1-57 firefly luciferase transcripts, which may reflect an effect of this pol sequence on mRNA accumulation.
FIG. 4.
Activity of the inhibitory element in transiently transfected cells. (A) Schematic diagram of constructs used in luciferase assays from transfected Vero cells highlighting the differences in the 5′ ends of these constructs. pGL3-MCS is a negative-control construct that contains the MCS of pBluescript as its 5′ leader. pGL3-pol5′UTR contains the complete 208-base pol 5′ leader upstream of the luciferase ORF. pGL3-pol1-57 and pGL3-pol58-208 contain bases 1 to 57 and 58 to 208 of the pol 5′ leader, respectively, upstream of the luciferase ORF. pGL3-pol-uORFmut contains the complete pol 5′ leader upstream of the luciferase ORF, but the AUG codon of the pol uORF has been mutated to AAG. (B) Luciferase assay data from transfected Vero cells. Vero cells were transfected with the constructs in panel A, each together with pRL-SV40, and infected with KOS at an MOI of 10 at 18 h posttransfection. At 6 hpi (24 h posttransfection), lysates were prepared and luciferase activity was determined. Results are presented as firefly luciferase activity normalized to Renilla luciferase activity to control for transfection and lysis efficiency and then normalized to firefly luciferase transcript levels that were previously normalized to Renilla luciferase transcript levels. Transcript levels were determined by real time RT-PCR as described in Materials and Methods (for data, see Supplemental Fig. 1 and 2 and Supplemental Table 1 at http://coen.med.harvard.edu/).
When luciferase activities were adjusted for transcript levels, we observed an approximately 20-fold decrease in normalized firefly luciferase activity per transcript from constructs containing the complete 208-base pol 5′ leader relative to that for the negative-control construct containing a 5′ leader of 176 bases derived from the pBluescript MCS (Fig. 4B, pol5′UTR compared to MCS). There was approximately a fivefold reduction when only the first ∼55 bases of the pol 5′ leader were present upstream of luciferase (Fig. 4B, pol1-57 compared to MCS). In agreement with the results from Fig. 1B, the remaining ∼150 bases of the pol 5′ leader had less than twofold inhibitory activity (Fig. 4B, pol58-208 compared to MCS) and destruction of the uORF by mutation of its initiation codon had no effect on alleviation of the inhibitory activity (Fig. 4B, uORFmut compared to pol5′UTR and MCS).
Similar results were obtained from mock-infected, transfected Vero cells and from in vitro translation of luciferase transcripts (data not shown). These results indicated that in addition to being necessary and sufficient to inhibit translation in vitro, the first ∼55 bases of the pol 5′ leader were also sufficient to inhibit translation in transfected cells that were infected with HSV-1. Our results also indicated that translation of the uORF in the pol 5′ UTR does not contribute to the observed inefficient translation of Pol, suggesting that relatively large deletions that remove this ORF and modestly increase translation, both in transfected cells and in vitro (Fig. 1 and (9), effect these increases by some other mechanism. Our results were in agreement with previous data from our laboratory, which showed that deletion of bases 57 to 200 from HSV, which leaves the inhibitory element intact but deletes the uORF, did not substantially increase the efficiency of Pol translation (29). We note that because total RNA was used to determine luciferase transcript levels, we cannot rule out the formal possibility that the observed changes in luciferase activity could be due to changes in nuclear export of the transcripts. However, because we saw similar results on translation in vitro in RRL, we think it highly likely that the differences in luciferase activity are due to differences in translational efficiency.
Importance of the inhibitory element to Pol expression during viral infection.
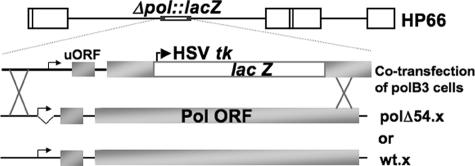
Since the first ∼55 bases of the pol 5′ leader were shown to be important for the inefficient translation of Pol in vitro and in transfected cells, we hypothesized that a mutant virus with this element deleted from the pol gene would overexpress Pol due to increased translation. To test this hypothesis, we generated a recombinant mutant virus by restoring pol in the pol-null virus HP66 (20) with the wild-type pol ORF sequence, but with the inhibitory element deleted from the pol 5′ leader (Fig. 5, polΔ54.x). We also generated a second recombinant virus by restoring pol in HP66 with the wild-type pol ORF sequence and the wild-type pol 5′ UTR (Fig. 5, wt.x).
FIG. 5.
Construction of a mutant deleted for an inhibitory element in the pol 5′ leader. Infectious DNA from the pol-null mutant virus HP66 (diagram shown in top line with relevant region shown in greater detail in the second line from top) and plasmids containing the wild-type pol ORF and either the wild-type pol 5′ UTR (bottom line) or the pol 5′ UTR with the inhibitory element deleted (third line from top) were transfected into polB3 cells, which express Pol and thus complement pol mutant viruses. Progeny from this cotransfection were then titrated on Vero cells to select for recombinants that have the pol ORF restored. Recombinants that formed plaques on Vero cells were subsequently screened for the presence of the pol 5′ UTR deletion by PCR. Correct isolates were plaque purified three times prior to their use in experiments.
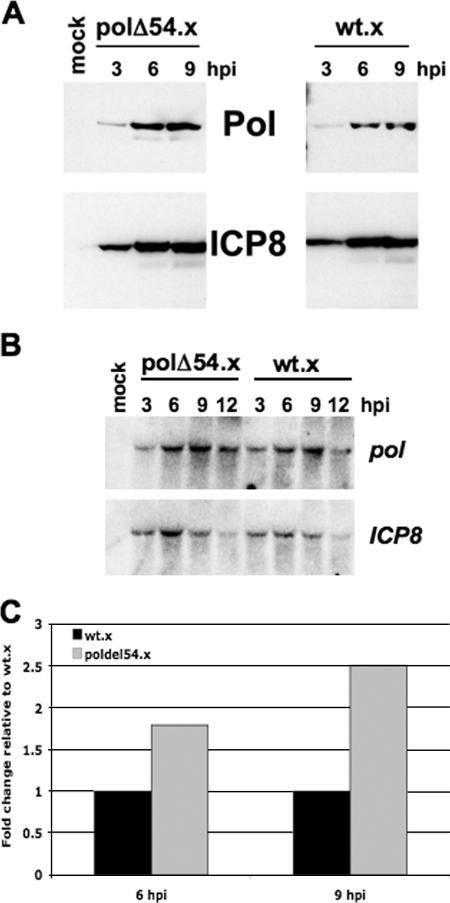
We compared the expression of Pol in cells infected with polΔ54.x to the expression of Pol in cells infected with wt.x by Western blotting (Fig. 6A) and quantified the protein levels by using serial dilutions of samples to generate a standard curve of the Western blot signals (see Supplemental Fig. 3 at http://coen.med.harvard.edu/). We observed that the levels of Pol relative to those of ICP8 were ∼2-fold higher in Vero cells infected with polΔ54.x than in those infected with wt.x at 9 hpi. To address the cause of Pol overexpression, pol and ICP8 transcript levels were determined by Northern blot analysis (Fig. 6B). There were equivalent amounts of RNA loaded in each lane, as indicated by the similar intensities of 28S and 18S ribosomal RNAs among the samples on the ethidium bromide-stained agarose gel prior to the transfer of these samples to the nylon membrane (data not shown). The results from quantification of the Northern blot data were used to adjust for any differences in pol and ICP8 transcript levels. Adjusted for mRNA accumulation, the accumulation of Pol relative to that of ICP8 was almost 2-fold higher at 6 hpi and 2.5-fold higher at 9 hpi in polΔ54.x-infected cells than in wt.x-infected cells (Fig. 6C). We considered the possibility that the polΔ54.x deletion resulted in increased nuclear export of pol mRNA. However, when we isolated RNA from nuclear and cytoplasmic fractions of infected cells, we found no difference in distribution of pol transcripts between polΔ54.x-infected cells and wt.x-infected cells, with roughly 50% of pol transcripts found in each fraction in both cases (unpublished results). Thus, Pol overexpression was, at least in part, a result of increased efficiency of Pol translation. This increase was not as great as that observed for luciferase activity relative to luciferase mRNA in transient-transfection assays. Possible explanations for the different magnitudes of effects observed in the different assays are explored in Discussion.
FIG. 6.
Regulation of Pol translation during HSV-1 infection. (A) Western blot analyses of Pol (top panels) and ICP8 (bottom panels) from Vero cells infected with either polΔ54.x (left panels), a mutant virus with the pol inhibitory element deleted, or wt.x (right panels), a control virus with the wild-type pol sequence, for the times indicated. Mock-infected Vero cell lysate was also run (lane 2). (B) Northern blot analysis of pol (top) and ICP8 (bottom) transcript levels during a time course of infection with either polΔ54.x or wt.x. (C) Quantification of the Western blot analyses performed by running a dilution series for both Pol and ICP8 to generate a standard curve that was used to determine the concentrations of Pol and ICP8 at 6 and 9 hpi in Vero cells infected with either wt.x (black bar) or polΔ54.x (gray bar) at an MOI of 10. Protein levels were also adjusted for differences in transcript levels from panel B. Values are presented as changes (n-fold) in Pol levels observed after normalization to ICP8 levels and adjustment for mRNA levels relative to the value for wt.x at each time point.
Overexpression of Pol is associated with decreased viral replication.
To determine whether the modest overexpression of Pol was accompanied by a change in the efficiency of viral replication, we performed multiple-cycle viral replication assays comparing polΔ54.x to the wt.x control virus. In these assays, polΔ54.x exhibited a modest replication defect of ∼6-fold relative to wt.x (3.0 × 106 ± 1.2 × 106 versus 1.9 × 107 ± 0.5 × 107 PFU/ml) at 72 hpi. These data draw a correlation between a modest increase in the efficiency of Pol translation and an accompanying modest replication defect.
DISCUSSION
We have shown in this report that at least part of the previously observed inefficient translation of Pol is mediated by an element in the 5′ leader of the pol transcript. The first ∼55 bases of the pol transcript, which have a G+C content of ∼80%, are both necessary and sufficient to inhibit translation in vitro, are sufficient to reduce translation in transfected Vero cells that were infected with HSV-1, and play a role in reducing translation of Pol during viral infection. RNase structure mapping of the inhibitory element allowed us to generate a model of the secondary structure of this RNA molecule which predicts that this RNA element forms regions that are double stranded and thus may inhibit translation by interfering with ribosomal loading and/or scanning. The modest overexpression of Pol in cells infected with an inhibitory element deletion mutant virus correlated with a modest replication defect of this mutant.
Potential mechanisms of inhibitory activity.
In principle, both the sequence and the structure of the inhibitory RNA element could contribute to its inhibitory activity. RNase structure mapping data of the first ∼55 bases of the pol 5′ leader reveal the existence of a secondary structure that could potentially be inhibitory to the initiation of translation. The model of the structure based on the RNase data has a stability of −16.7 kcal/mol, and translation of other mRNAs has been shown to be inhibited by RNA structures with similar stabilities (2). The longest and most stable stretch of double-stranded RNA is also the stretch of double-stranded RNA located closest to the 5′ end of the transcript, and close proximity to the cap has been shown to enhance the inhibitory activities of RNA structures in other genes (12, 16). Removal of bases at the extreme 5′ end of the 5′ UTR, which are predicted by the model to be unstructured, moves the most stable predicted structure closer to the 5′ cap and also results in increased inhibitory activity, thus providing evidence for a role for RNA structure in the inhibitory mechanism. However, our data do not permit us to conclude that RNA structure alone is responsible for the inhibition of translation. Additional experiments will be required to elucidate the inhibitory mechanism of this RNA element.
Regulation of Pol translation during HSV-1 infection.
After characterizing the inhibitory element in the pol 5′ leader in vitro and in transfected cells, we tested whether this element was also able to regulate the translation of Pol during HSV-1 infection. To address this question, we constructed a recombinant mutant virus with the inhibitory element deleted from the pol 5′ leader and measured the effect of this mutation on Pol translation and viral replication.
Deletion of the inhibitory element from the viral genome resulted in a modest overexpression of Pol, and accumulation of Pol relative to pol mRNA was increased ∼2- to 3-fold in cells infected with the deletion mutant viruses relative to that in cells infected with the control virus. This indicates that the inhibitory element that we discovered in vitro is also involved in regulating Pol translation during HSV-1 infection. Although deletion of the inhibitory element results in Pol overexpression, the increased accumulation of Pol was not nearly as great as what we observed for increases in luciferase activity relative to luciferase mRNA in transfected, infected cells when comparing the construct containing the full-length pol 5′ UTR with the construct with the inhibitory element deleted (pol58-208). There, the difference was ∼10-fold. The increased accumulation of Pol was also not as great as the differences observed between the rate of translation of Pol per pol mRNA and the rate of translation of thymidine kinase per tk mRNA at 6 to 9 hpi, which were 6- to 14-fold (27, 29). The inhibitory element also exerted 5- to 10-fold effects in RRL in vitro when either radiolabeled Pol or luciferase activity was used as a reporter. Some of these differences may relate to parameters of the assays involved, e.g., the relative stabilities of Pol versus luciferase and RRL versus cultured cells. Nevertheless, we hypothesize that there may be a layer of regulation involved in downregulating the levels of Pol during infection aside from the inhibitory element in the pol 5′ UTR. Because we observed ∼10-fold effects of the inhibitory element on luciferase expression in HSV-infected, transfected cells, we also hypothesize that an additional layer of regulation would involve pol sequences downstream of the 5′ UTR.
One attractive possibility for this additional layer is autogenous regulation of Pol translation, which is a mechanism that has been observed to regulate the expression of other viral DNA polymerases, such as those encoded by bacteriophage T4 and related phages (1). In this scenario, when levels of Pol increase during infection, excess Pol could bind to the pol transcript and inhibit translation until the levels of Pol drop. The recently solved crystal structure of HSV-1 Pol revealed that the enzyme contains a ribonucleoprotein (RNP) motif (19), suggesting a mechanism by which Pol could bind its own mRNA to result in autogenous regulation of translation. RNP motifs are well known to be involved in RNA binding (4), and in addition to the HSV-1 Pol, several other α-family DNA Pols, including some known to autogenously regulate their translation, have also been shown to contain RNP motifs (11, 24-26, 31). Autogenous regulation of Pol translation may be an alternate mechanism used by the virus to keep Pol levels low when other mechanisms, such as the inhibitory element in the pol 5′ leader, are compromised. Autogenous regulation may also explain the previous observation that Pol translation is shut off at approximately 6 hpi, although pol transcript levels are high at this time (27, 29). Perhaps during HSV-1 infection, Pol is expressed to high enough levels by this time to inhibit further translation at later time points. Additional experiments will be necessary to determine whether autogenous regulation of Pol translation occurs during HSV-1 infection or whether other mechanisms that downregulate Pol expression are operating.
Increased translation of Pol correlates with a modest HSV-1 replication defect.
The mutant virus with the inhibitory element deleted has a modest replication defect relative to a control virus with the wild-type sequence. We suggest that this modest replication defect is likely to be due to the mutant's modestly elevated levels of Pol. Multiple other viral and cellular polymerases are expressed at low levels (reviewed in reference 7), and in the case of some viruses, overexpression of viral polymerases has been shown to result in a decrease in viral replication (22).
One model that could explain this result is that HSV-1 may require levels of Pol to remain relatively low to avoid formation of counterproductive interactions between Pol and other limiting components of the viral DNA replication complex. If Pol levels were to become too high, Pol may form interactions with only a subset of the other proteins necessary to replicate viral DNA, effectively sequestering these other proteins, preventing them from forming complete DNA replication complexes. Pol has been reported to interact and/or colocalize with viral proteins involved in HSV-1 DNA replication, such as UL42 (8), UL8 (21), and ICP8 (5), and thus, these proteins are candidates for potential sequestration by Pol.
Alternatively, viral polymerases may contain epitopes recognized by the immune system of their host. Therefore, overexpression of viral polymerases may result in an increased immune response. There is some evidence suggesting that downregulating the expression of a viral polymerase helps the virus avoid recognition by the immune system (23, 30). This model could explain the evolution of mechanisms for down-regulation of Pol expression but would not explain a role for decreased Pol expression during viral replication in cell culture.
The results presented here begin to dissect the mechanism involved in regulating the translation of Pol and investigate the importance of this regulation during viral replication. These results also provide a framework for subsequent experiments for further study of these issues.
Acknowledgments
We thank David Knipe, Blair Strang, Anthony Griffiths, Angela Pearson, and Lee Gehrke for helpful discussions, David Knipe for providing the 3-83 ICP8 antibody and the plasmid pSP8pA, Louane Hann for providing the plasmid LEH.VII.5W.13 and for previous work on this project, John Balliet and Priscilla Schaffer for providing the plasmid pWR.3, Dorne Yager for early work on this project, and Christine Nguyen for constructing the plasmids pWR.3 (-BamHI), p3WB2, and pW1ΔB2, for designing the PCR assay for screening for inhibitory element deletions, and for assistance with preparation of figures. Use of the Wallac Victor 2 luminometer was kindly provided by the Harvard Medical School Institute of Chemistry and Cellular Biology.
This work was supported by NIH grant AI19838 awarded to D.M.C. K.F.B. was supported, in part, by Viral-Host Interactions in Cancer training grant T32 CA09031.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Andrake, M., N. Guild, T. Hsu, L. Gold, C. Tuerk, and J. Karam. 1988. DNA polymerase of bacteriophage T4 is an autogenous translational repressor. Proc. Natl. Acad. Sci. USA 857942-7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baim, S. B., and F. Sherman. 1988. mRNA structures influencing translation in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 81591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balliet, J. W., J. C. Min, M. S. Cabatingan, and P. A. Schaffer. 2005. Site-directed mutagenesis of large DNA palindromes: construction and in vitro characterization of herpes simplex virus type 1 mutants containing point mutations that eliminate the oriL or oriS initiation function. J. Virol. 7912783-12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burd, C. G., and G. Dreyfuss. 1994. Conserved structures and diversity of functions of RNA-binding proteins. Science 265615-621. [DOI] [PubMed] [Google Scholar]
- 5.Bush, M., D. R. Yager, M. Gao, K. Weisshart, A. I. Marcy, D. M. Coen, and D. M. Knipe. 1991. Correct intranuclear localization of herpes simplex virus DNA polymerase requires the viral ICP8 DNA-binding protein. J. Virol. 651082-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiou, H. C., S. K. Weller, and D. M. Coen. 1985. Mutations in the herpes simplex virus major DNA-binding protein gene leading to altered sensitivity to DNA polymerase inhibitors. Virology 145213-226. [DOI] [PubMed] [Google Scholar]
- 7.Coen, D. M. 1996. Viral DNA polymerases, p. 495-523. In M. L. DePamphilis (ed.), DNA replication in eukaryotic cells. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 8.Digard, P., and D. M. Coen. 1990. A novel functional domain of an alpha-like DNA polymerase. The binding site on the herpes simplex virus polymerase for the viral UL42 protein. J. Biol. Chem. 26517393-17396. [PubMed] [Google Scholar]
- 9.Dorsky, D. I., and C. S. Crumpacker. 1988. Expression of herpes simplex virus type 1 DNA polymerase gene by in vitro translation and effects of gene deletions on activity. J. Virol. 623224-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison, K. S., R. A. Maranchuk, K. L. Mottet, and J. R. Smiley. 2005. Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J. Virol. 794120-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin, M. C., J. Wang, and T. A. Steitz. 2001. Structure of the replicating complex of a pol alpha family DNA polymerase. Cell 105657-667. [DOI] [PubMed] [Google Scholar]
- 12.Goossen, B., and M. W. Hentze. 1992. Position is the critical determinant for function of iron-responsive elements as translational regulators. Mol. Cell. Biol. 121959-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 148-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang, Y. T., B. Y. Liu, D. M. Coen, and C. B. Hwang. 1997. Effects of mutations in the Exo III motif of the herpes simplex virus DNA polymerase gene on enzyme activities, viral replication, and replication fidelity. J. Virol. 717791-7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knipe, D. M., D. Senechek, S. A. Rice, and J. L. Smith. 1987. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J. Virol. 61276-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak, M. 1989. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol. Cell. Biol. 95134-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozak, M. 2005. Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene 36113-37. [DOI] [PubMed] [Google Scholar]
- 18.Kramer, M. F., and D. M. Coen. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 691389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, S., J. D. Knafels, J. S. Chang, G. A. Waszak, E. T. Baldwin, M. R. Deibel, Jr., D. R. Thomsen, F. L. Homa, P. A. Wells, M. C. Tory, R. A. Poorman, H. Gao, X. Qiu, and A. P. Seddon. 2006. Crystal structure of the herpes simplex virus 1 DNA polymerase. J. Biol. Chem. 28118193-18200. [DOI] [PubMed] [Google Scholar]
- 20.Marcy, A. I., D. R. Yager, and D. M. Coen. 1990. Isolation and characterization of herpes simplex virus mutants containing engineered mutations at the DNA polymerase locus. J. Virol. 642208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsden, H. S., G. W. McLean, E. C. Barnard, G. J. Francis, K. MacEachran, M. Murphy, G. McVey, A. Cross, A. P. Abbotts, and N. D. Stow. 1997. The catalytic subunit of the DNA polymerase of herpes simplex virus type 1 interacts specifically with the C terminus of the UL8 component of the viral helicase-primase complex. J. Virol. 716390-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier, E., G. G. Harmison, and M. Schubert. 1987. Homotypic and heterotypic exclusion of vesicular stomatitis virus replication by high levels of recombinant polymerase protein L. J. Virol. 613133-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehermann, B., P. Fowler, J. Sidney, J. Person, A. Redeker, M. Brown, B. Moss, A. Sette, and F. V. Chisari. 1995. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J. Exp. Med. 1811047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez, A. C., H. W. Park, C. Mao, and L. S. Beese. 2000. Crystal structure of a pol alpha family DNA polymerase from the hyperthermophilic archaeon Thermococcus sp. 9 degrees N-7. J. Mol. Biol. 299447-462. [DOI] [PubMed] [Google Scholar]
- 25.Shamoo, Y., and T. A. Steitz. 1999. Building a replisome from interacting pieces: sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex. Cell 99155-166. [DOI] [PubMed] [Google Scholar]
- 26.Wang, J., A. K. Sattar, C. C. Wang, J. D. Karam, W. H. Konigsberg, and T. A. Steitz. 1997. Crystal structure of a pol alpha family replication DNA polymerase from bacteriophage RB69. Cell 891087-1099. [DOI] [PubMed] [Google Scholar]
- 27.Wobbe, K. K., P. Digard, D. Staknis, and D. M. Coen. 1993. Unusual regulation of expression of the herpes simplex virus DNA polymerase gene. J. Virol. 675419-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yager, D. R., and D. M. Coen. 1988. Analysis of the transcript of the herpes simplex virus DNA polymerase gene provides evidence that polymerase expression is inefficient at the level of translation. J. Virol. 622007-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yager, D. R., A. I. Marcy, and D. M. Coen. 1990. Translational regulation of herpes simplex virus DNA polymerase. J. Virol. 642217-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao, E., and J. E. Tavis. 2003. Kinetics of synthesis and turnover of the duck hepatitis B virus reverse transcriptase. J. Biol. Chem. 2781201-1205. [DOI] [PubMed] [Google Scholar]
- 31.Zhao, Y., D. Jeruzalmi, I. Moarefi, L. Leighton, R. Lasken, and J. Kuriyan. 1999. Crystal structure of an archaebacterial DNA polymerase. Structure 71189-1199. [DOI] [PubMed] [Google Scholar]
- 32.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 313406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]