TABLE 3.
Group comparisons of measured parametersa
| Parameter | Analysis | Unit of analysis | Subgroup 1
|
Subgroup 2
|
Test | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup name | n | Median | IQR | Subgroup name | n | Median | IQR | |||||
Total plasma NT70 (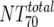 ) ) |
I | Heterologous virus NT70 | Pretreatment | 14 | 40 | 40-84 | Wk 12 | 14 | 482 | 342-578 | W | 0.0001 |
| II | Heterologous virus NT70 | Wk 12 | 13b | 436 | 342-551 | Wk 24 | 13 | 113 | 55-154 | W | 0.0046 | |
| III | Heterologous virus NT70 | Responders | 6 | 501 | 373-553 | Nonresponders | 8 | 361 | 327-667 | M | 0.491 | |
| IV | Autologous virus NT70 | Responders | 6 | 732 | 175-1,808 | Nonresponders | 8 | 234 | 147-703 | M | 0.414 | |
MAb NT70 (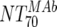 ) ) |
V | Heterologous virus NT70 |  |
14 | 350 | 205-455 |  |
14 | 78 | 40-122 | M | <0.0001 |
| VI | Heterologous virus NT70 | 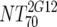 |
14 | 350 | 205-455 | 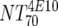 |
14 | 30 | 18-44 | M | <0.0001 | |
| VII | Heterologous virus NT70 | Responders | 6 | 514 | 468-618 | Nonresponders | 8 | 317 | 260-552 | M | 0.181 | |
| VIII | Autologous virus NT70 | Responders | 6 | 614 | 192-1,520 | Nonresponders | 8 | 95 | 35-149 | M | 0.0127 | |
| IX | Autologous virus NT70; pretreatment | Acute | 6 | 40 | 40-40 | Chronic | 8 | 110 | 93-243 | NAc | ||
| X | Autologous virus NT70; wk 24 | Acute | 6 | 168 | 51-455 | Chronic | 7 | 178 | 160-2,062 | M | 0.366 | |
Autologous-Ab NT70 (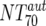 ) ) |
XI | Autologous virus NT70 | Responders | 6 | 10 | 10-1,036 | Nonresponders | 8 | 165 | 85-399 | M | 0.0813 |
| Fold difference | XII | 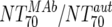 |
Responders | 6 | 43.3 | 2.74-152 | Nonresponders | 8 | 0.63 | 0.07-16.2 | M | 0.0293 |
| XIII | 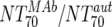 |
Responders without NAB14d | 5 | 74.2 | 8.9-152 | Nonresponders | 8 | 0.63 | 0.07-16.2 | M | 0.0062 | |
IQR, interquartile range; W, Wilcoxon signed rank test; M, Mann-Whitney U test; NA, not applicable; Ab, antibody.
Patient NAB06 reinititated antiretroviral therapy after week 12; thus, data from only 13 patients are available for group comparison of weeks 12 and 24.
Group comparison not applicable, because at the pretreatment time point, no neutralization activity was detectable in acute infection; the NT range for chronic infection was 71 to 965.
Patient NAB14 was excluded from the analysis.
