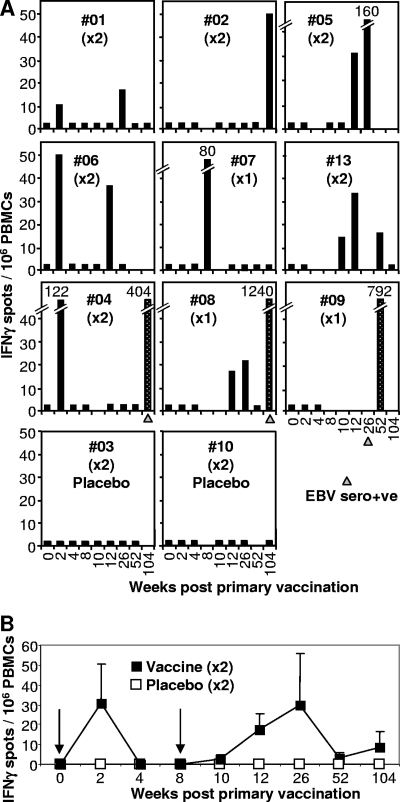
FIG. 1.
Ex vivo IFN-γ ELISPOT analysis. (A) The vaccine was administered twice at week 0 and week 8 (×2), except for vaccinees #07, #08, and #09, who received only the first vaccination (×1). Vaccinees received a 5-μg dose of peptide, except #13, who received a 50-μg dose, and #03 and #10, who received no peptide (placebo). PBMC from vaccinees collected prior to vaccination (week 0) and at the indicated number of weeks after the first vaccination were analyzed by ex vivo IFN-γ ELISPOT assay using the FLR peptide and frozen PBMC. Three vaccinees seroconverted asymptomatically within 2 years (#04, #08, and #09), and the time at which serology first indicated EBV seroconversion is indicated with a gray triangle. Vaccine-induced responses are represented by black bars. Speckled bars illustrate responses after EBV seroconversion. Black bars at 2.5 IFN-γ spots/106 PBMC represent no significant response and are shown to indicate that an assay was performed on PBMC collected at that time point. (B) Mean numbers of IFN-γ spots (± standard errors) for vaccinees #01, #02, #04, #05, #06, and #13 (black squares), who received vaccinations at 0 and 8 weeks (arrows). The mean numbers of spots for all four placebo recipients are also shown (white squares). The two groups are significantly different (P = 0.041) by analysis of variance, which included a term for weeks.