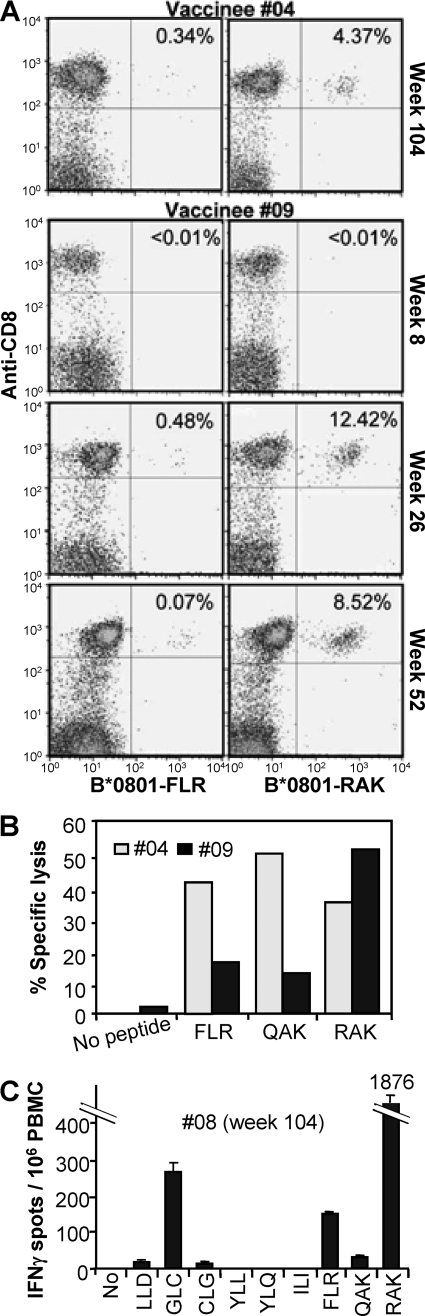
FIG. 4.
CD8 T-cell response of vaccinees after EBV seroconversion. (A) PBMC from vaccinees (no. 9 and 4) collected at the indicated time points postvaccination were assessed by FACS using FLR-specific (left panels, B*0801-FLR) and RAK-specific (right panels, B*0801-RAK) pentamers (x axis) and anti-CD8 monoclonal antibody (y axis). The percentage of CD8 T cells that are pentamer positive are indicated in the top right corner of each panel. (B) Standard chromium release assay using bulk cultures generated from PBMC from vaccinees #09 (week 52) and #04 (week 104) to asses lytic activity specific for FLR, QAK, and RAK. (C) Ex vivo IFN-γ ELISPOT assay using PBMC from vaccinee #08 (collected at week 104) to asses T-cell responses to the HLA A*0201-restricted epitopes LLDFVRFMGV (LLD), GLCTLVAML (GLC), CLGGLLTMV (CLG), ILIYNGWYA (ILI), YLLEMLWRL (YLL), and YLQQNWWTL (YLQ) and the HLA B*0801-restricted epitopes FLR, QAK, and RAK.