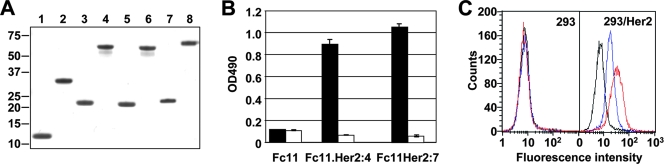
FIG. 2.
Molecular properties of the Fc11-affibody chimeras. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified recombinant proteins. The Fc11 backbone (lanes 1 and 2), its affibody-modified derivatives Fc11.Her2:7 (lanes 3 and 4) and Fc11.Her2:4 (lanes 5 and 6), and Ad5 fiber knob for comparison (lanes 7 and 8) purified from bacterial cells were loaded on the gel with or without prior denaturation by boiling (odd-numbered and even-numbered lanes, respectively). While the proteins in the denatured samples are monomeric polypeptides, the proteins in the nondenatured samples are trimers. The positions and molecular masses (in kDa) of protein standards are shown on the left. (B) Binding of affibody-containing proteins to recombinant Her2. Purified ErbB2/Fc protein immobilized on an enzyme-linked immunosorbent assay plate (500 ng per well) was probed with the Fc11 backbone, Fc11.Her2:7, or Fc11.Her2:4 (black bars) followed by anti-His5 MAb and secondary antibody-horseradish peroxidase conjugate. The white bars indicate background binding to casein. Error bars indicate the standard deviations obtained in triplicate samples. OD490, optical density at 490 nm. (C) Target receptor-selective attachment of the affibody-containing proteins to Her2-positive cells. Fc11 (black), Fc11.Her2:7 (red), and Fc11.Her2:4 (blue) were used to probe the surface of Her2-negative 293 cells and Her2-expressing 293/Her2 cells.