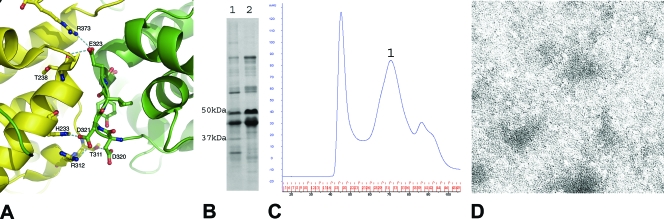
FIG. 4.
(A) Cartoon illustration for a stretch of residues (residues 320 to 324) involved in side-by-side interactions. These residues form five hydrogen bonds with the neighboring N molecule in the oligomeric N-RNA complex, including those between the side chain of Asp320 and the side chain of Thr311; the side chain of Asp321 and the side chain of His233, as well as that of Arg312; the side chain of Asp323 and the carbonyl of Thr238; and the side chain of Thr325 and the side chain of Arg309. The side chain of Tyr309 fits into a hydrophobic pocket on the surface of the C-lobe of the neighboring N molecule. (B) SDS-PAGE stained with Coomassie blue, showing the coexpression and copurification of N (320-324, (Ala)5) with the His-tagged P protein. Lane 1, void volume peak; lane 2, peak 1 (peak numbering refers to the peaks in panel C). (C) The size exclusion chromatography profile of the N (320-324, (Ala)5)-P protein complex after nickel-affinity purification. Peaks are labeled sequentially. The peak before peak 1 corresponds to the void volume. (D) Conventional transmission electron micrograph of the sample represented by peak 1. The sample was prepared at a concentration of 0.05 mg/ml protein and was stained with 2% aqueous uranyl acetate. The micrograph was taken at a magnification of ×50,000.