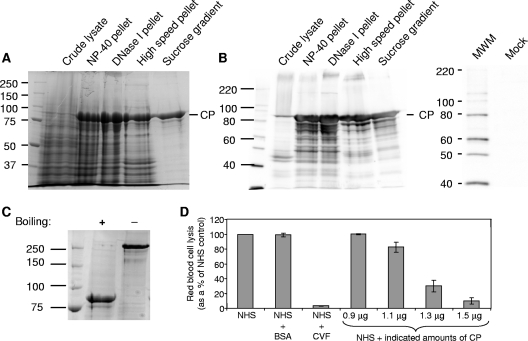
FIG. 4.
Analysis of HAstV-1 CP purification, oligomerization state, and activity in the hemolytic complement assay. Coomassie blue staining (A) and immunoblot analysis (B) of the CP-containing fraction were performed at each stage of the purification procedure. An IPLB-Sf21 mock-infected cellular lysate was also analyzed by immunoblotting to demonstrate that there was no cross-reactivity to the HAstV-1 antibody. Molecular size markers (MWM; in kDa) are indicated to the left, with the position of CP denoted on the right. (C) Aliquots of sucrose-purified CP were either boiled or not boiled in the presence of 2-mercaptoethanol, resolved by SDS-PAGE, and then stained with Coomassie blue. The boiled protein migrated at 87 kDa, the expected mass of the uncleaved CP precursor (monomer), whereas the unboiled sample migrated above 250 kDa, possibly representing a trimer. Molecular size markers (in kDa) are indicated to the left. (D) Antibody-sensitized sheep RBCs were incubated with 2% NHS alone or in the presence of 15 μg BSA, 1 μg CVF, or the indicated amounts of purified CP. Hemolysis was standardized to 100% for NHS alone. Data are the means for four independent experiments. Error bars denote SEM.