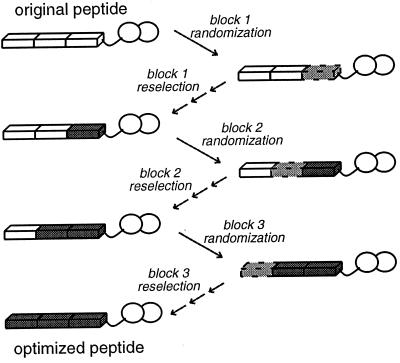
Figure 3.
Overall scheme for sequential reoptimization of peptides. A 15-residue peptide obtained from the initial selection was divided conceptually into three blocks of 5 aa each and reoptimized in three steps. In the first step, the five-residue block closest to the fingers was completely randomized, with the other 10 aa held constant. Phage display of the new fusion proteins, with six to nine selection and amplification cycles, was used to obtain the best sequences from this pool. In the second reoptimization step, the central five-residue block was completely randomized, with the finger-proximal region held constant as the newly optimized sequence and the finger-distal region corresponding to the initially selected sequence. The best sequences from this pool were obtained again via phage display with a series of selection and amplification cycles. In the final reoptimization step, the finger-distal five-residue block was completely randomized and then reselected in the context of the two other reoptimized blocks.