Table 1. Crystallographic data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Data | PmSOD1 | PmSOD2 |
|---|---|---|
| Resolution (Å) | 56.8–1.86 (1.93–1.86) | 30–2.5 (2.57–2.5) |
| Rmerge† (%) | 4.6 (29.5) | 8.6 (33.0) |
| Completeness (%) | 95 (91) | 96.7 (92.9) |
| Redundancy | 5.5 (4.0) | 8.0 (3.0) |
| I/σ(I) | 21.6 (4.1) | 20.0 (2.6) |
| Refinement statistics | ||
| R factor‡ (%) | 17.1 (24.8) | 19.7 (18.5) |
| Rfree§ | 21.6 (26.2) | 27.6 (33.9) |
| No. of atoms | ||
| Protein | 3147 | 3211 |
| Water | 408 | 203 |
| Fe | 2 | 2 |
| Mean B factor (Å2) | 16.8 | 15.1 |
| R.m.s. deviations from ideal | ||
| Bond lengths (Å) | 0.014 | 0.015 |
| Bond angles (°) | 1.3 | 1.7 |
| Chiral (Å3) | 0.108 | 0.099 |
R
merge = 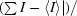
 , where I is the observed intensity and 〈I〉 is the average intensity obtained from multiple observations of symmetry-related reflections after rejections.
, where I is the observed intensity and 〈I〉 is the average intensity obtained from multiple observations of symmetry-related reflections after rejections.
R factor = 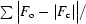
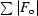 , where F
o are observed and F
c are calculated structure factors.
, where F
o are observed and F
c are calculated structure factors.
The R free set uses 5% of randomly chosen reflections.
