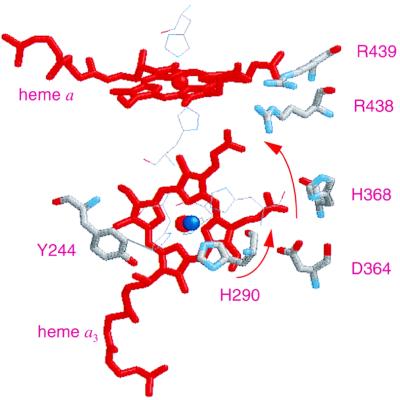
Figure 4.
Structural model of the redox centers in A. ambivalens aa3 oxidase. The model was created based on the structural coordinates of Paracoccus cytochrome c oxidase, taking only the first 491 residues of the amino acid sequence into consideration. The residues are numbered as in bovine sequence. Shown in the picture are heme a, heme a3 (red), CuB (blue), and some of the neighboring residues (in thick lines), His-290 (one of the three histidine ligands to CuB), Tyr-244, Asp-364, His-368, Arg-438, and Arg-439. The residues shown in thin lines but not labeled are the histidine ligands to hemes a and a3 and CuB. The arrows denote the proposed path of proton translocation from His-290 to the Asp-364–propionate pair to the Arg-438–propionate region.