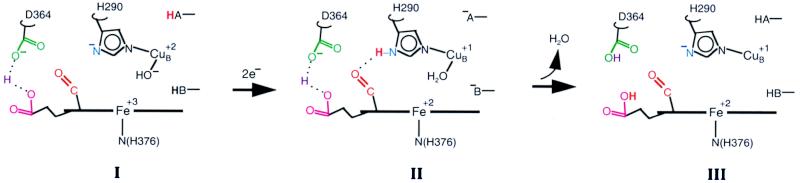
Figure 5.
Model of proton pumping. The residues are numbered following the sequence of bovine aa3. The sequence of structures shown is I, the oxidized form; II, the transient reduced form; and III, the final reduced form. Only selective residues around the heme a3-CuB site are shown. Two unidentified groups capable of donating protons are represented as −BH and −AH. The red proton shown in bold denotes the translocable proton. Asp-364 and the propionate share the proton placed in between them. In the oxidized form (structure I), the formyl of a3 is not hydrogen-bonded as the H290 side chain is an imidazolate. Upon reduction, the formyl group forms hydrogen bonding transiently with the −NδH of imidazole (structure II). In structure III, the hydrogen bond is broken again and the proton is transferred to the propionate–Asp-364 pair.