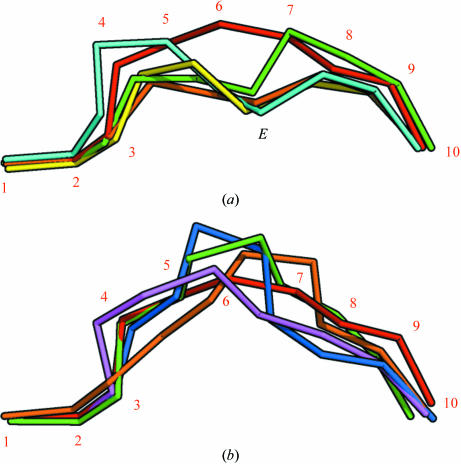
Figure 4.
The peptide positions in the binding groove after superimposition of MHC complex structures. The Cα atoms of the peptides have been connected and α-chain residues have been removed for clarity. (a) The AIMPARFYPK peptide (red, numbered) follows a relatively superficial path, whereas other decamer peptides bound by A3 supertype molecules [green, HLA-A*6801 complex (PDB code 1tmc; Collins et al., 1995 ▶); cyan, HLA-A*1101 complex (PDB code 1qvo; Li & Bouvier, 2004 ▶)] achieve a deeper position by placing residue 6 in the E pocket (indicated with an E). Complexes with nonamer peptides [yellow, HLA-A*1101 complex (PDB code 1q94; Li & Bouvier, 2004 ▶); green, HLA-A*1101 complex (PDB code 1x7q; Blicher et al., 2005 ▶)] utilize residue 5 for binding in the E pocket in a similar fashion. (b) Comparison of the AIMPARFYPK peptide (red) with two decamer peptides [purple, HLA-A*0201 complex (PDB code 1i4f; Hillig et al., 2001 ▶); orange, HLA-B*2705 (PDB code 2bss; Stewart-Jones, Di Gleria et al., 2005 ▶)] and two 11-residue peptides [blue, HLA-B*5703 (PDB code 2bvo; Stewart-Jones, Gillespie et al., 2005 ▶); green, HLA-B*3501 (PDB code 1zsd; Miles et al., 2005 ▶)] bound by other HLA alleles. Although the AIMPARFYPK peptide bulges out of the groove, it is not unusual for a decamer peptide. The orientation is the same as in (a).