Table 4. Peptide-binding interactions.
| Peptide | Hydrogen-bond partner† | ||||
|---|---|---|---|---|---|
| Residue | Atom | Residue | Atom | Distance (Å) | van der Waals contact residues‡ |
| Ala1p | N | Tyr7α | OH | 3.0 | Met5α, Tyr7α, Glu63α, Trp167α |
| N | Tyr171α | OH | 2.7 | ||
| O | Tyr159α | OH | 2.5 | ||
| Ile2p | N | Glu63α | O∊1 | 2.9 | Tyr7α, Tyr9α, Met45α, Glu63α, Asn66α, Val67α, Tyr99α, Tyr159α, W69 |
| O | W69 | OW | 3.0 | ||
| Met3p | N | Tyr99α | OH | 2.9 | Asn66α, Tyr99α, Arg114α, Gln156α, Tyr159α, (W215) |
| Pro4p | O | W384 | OW | 2.7 | Tyr159α, (SO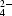 4003), (W179), (W340) 4003), (W179), (W340) |
| Ala5p | N | Asn66α | Oδ1 | 3.4 | (Asn66α), (W189), (W384) |
| Arg6p | N | W384 | OW | 3.1 | Gln155α, Tyr8p |
| O | W41 | OW | 3.5 | ||
| O | W167 | OW | 2.7 | ||
| O | (W206) | OW | 3.5 | ||
| N∊ | (Tyr8p) | OH | 3.0 | ||
| Nη2 | (Tyr8p) | OH | 3.0 | ||
| Nη1 | (W340) | OW | 2.7 | ||
| Phe7p | N | (W377) | OW | 2.9 | Thr73α, Val76α, Pro9p, (W41) |
| Tyr8p | N | W41 | OW | 2.9 | Trp147α, Trp147α, Ala150α, Ala152α, Gln155α, Arg6p, (W41), (W62), (W125), (W167), (W210) |
| O | (W41) | OW | 3.4 | ||
| O | (W45) | OW | 2.6 | ||
| O | (Trp147α) | N∊1 | 3.0 | ||
| Pro9p | O | (W62) | OW | 2.7 | Asp77α, Lys146α, Trp147α, Phe7p, W11, W62, W91, (W187), (W291), (W468) |
| O | (Trp147α) | N∊1 | 3.3 | ||
| Lys10p | N | Asp77α | Oδ1 | 3.0 | Asp77α, Leu81α, Ile95α, Thr143α, Trp147α, W45 |
| OXT | Tyr84α | OH | 2.6 | ||
| OXT | Thr143α | Oγ1 | 2.7 | ||
| O | Lys146α | Nζ | 2.7 | ||
| O | W11 | OW | 2.9 | ||
| Nζ | Asp116α | Oδ2 | 2.7 | ||
| Nζ | W2 | OW | 2.9 | ||
| Nζ | W6 | OW | 2.9 | ||
The cutoff distance for a hydrogen bond is 3.5 Å. Entities in parentheses form hydrogen bonds with suboptimal geometry (polar contact) or are judged not to be important for peptide binding.
The cutoff distance for a van der Waals contact is 4.0 Å. Residues having any atom within this distance are considered to be contact residues. Entities in parentheses are judged not to be important for peptide binding.
