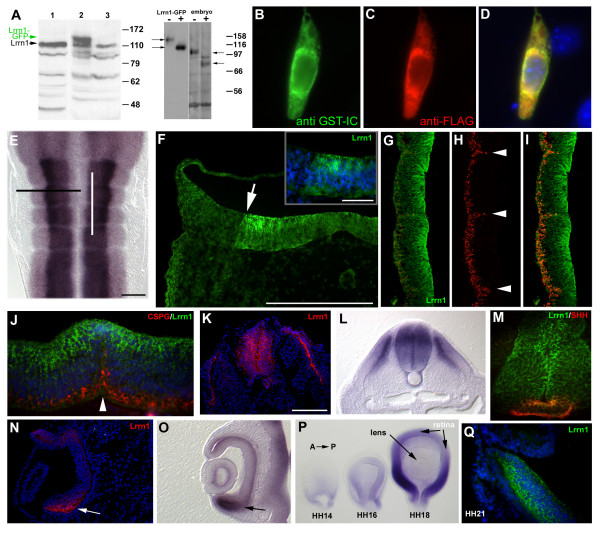
Figure 5.
Distribution of the Lrrn1 protein. (a) Western blot analysis using the GST-IC antiserum. Left panel: whole-cell protein lysate from whole embryo (HH18, lane 1), or chick embryo fibroblasts (CEFs) transfected with a vector that expresses a Lrrn1-GFP fusion protein (lane 2), or empty vector control (lane 3). GST-IC recognizes a predominant species at 110 kDa (Lrrn1) also present in CEFs. The Lrrn1-GFP fusion protein runs at the expected size, approximately 27 kDa larger, at 140 kDa (green arrow). Right panel: control lysate (-) or lysate treated with PNGase F (+) from Lrrn1-GFP transfected Chinese hamster ovary (CHO) cells or HH18 embryos. In each case a shift of approx 30 kDa is seen after endoglycosidase treatment. Position of molecular weight protein standards is shown to the right in each case. (b-d) Epifluorescent images of a Neuro2a cell stained immunocytochemically following transfection (24 h) with a mouse FLAG-Lrrn1 construct and stained (b) with the GST-IC antiserum in green, (c) or an anti-FLAG antibody in red. (d) Merged image shows colocalisation of the two immunoreactivities (yellow). Nuclei are counterstained with Hoechst 33342 (blue). (e) Flatmount of HH17 hindbrain hybridised in situ for Lrrn1 as shown in Figure 3a. Black line indicates plane of section in (f). White line indicates plane of section in (g-i). (f) Lrrn1 immunohistochemistry with GST-IC shows strong staining in the ventricular layer of the hindbrain with a clear dorsoventral limit of expression (white arrow). Speckled, endosomal-like staining in NPCs in the ventricular region is shown at higher magnification (inset). Nuclei are counterstained with Hoechst 33342 (blue). (g-i) Longitudinal section through the hindbrain at HH17 stained with GST-IC (g) and the anti-neurofilament antibody RMO-270 (h), which labels axonal processes. Anterior is uppermost. (i) Merged image shows absence of colocalisation of the two markers, particularly at rhombomere boundaries (arrowheads in (h)). (j) Longitudinal section through the r4–5 boundary at HH18 double stained for Lrrn1 (green), chondroitin sulphate proteoglycan (CSPG using the CS-56 antibody, red) and counterstained with Hoechst 33342 (blue). Lrrn1 immunostaining is visible at the most apical region of the boundary (white arrow head) but not in more basal regions. (k) Cryostat section taken transversely through HH18 spinal chord stained for Lrrn1 (red). Hoechst 33342 counterstained nuclei are shown in blue. Note, congruence between Lrrn1 immunostaining and distribution of Lrrn1 RNA as shown by in situ hybridisation in a comparable section from a similar specimen (l). Double labelling with Lrrn1 and SHH (5E1 antibody) shows that Lrrn1 is largely absent from the floorplate but some overlap exists in the apical region. (n) Cryostat section taken transversely through HH18 eye stained for Lrrn1 (red). Strong staining (white arrow) correlates strictly with strong staining for Lrrn1 by in situ from a similar specimen ((o) black arrow). (p) Optic cup/eye from HH14–18 specimens hybridised in situ for Lrrn1 (anterior to the right). Expression is seen in posterior, ventral-strong to anterior, dorsal-weak gradient. Transverse section through posterior, ventral part of the eye of a HH21 embryo stained for Lrrn1. Note, similar distribution of intensely labelled speckles in the retina as seen in other regions and absence of staining in the lens. Scale bars = 10 μm except in the inset of (f) = 5μm.